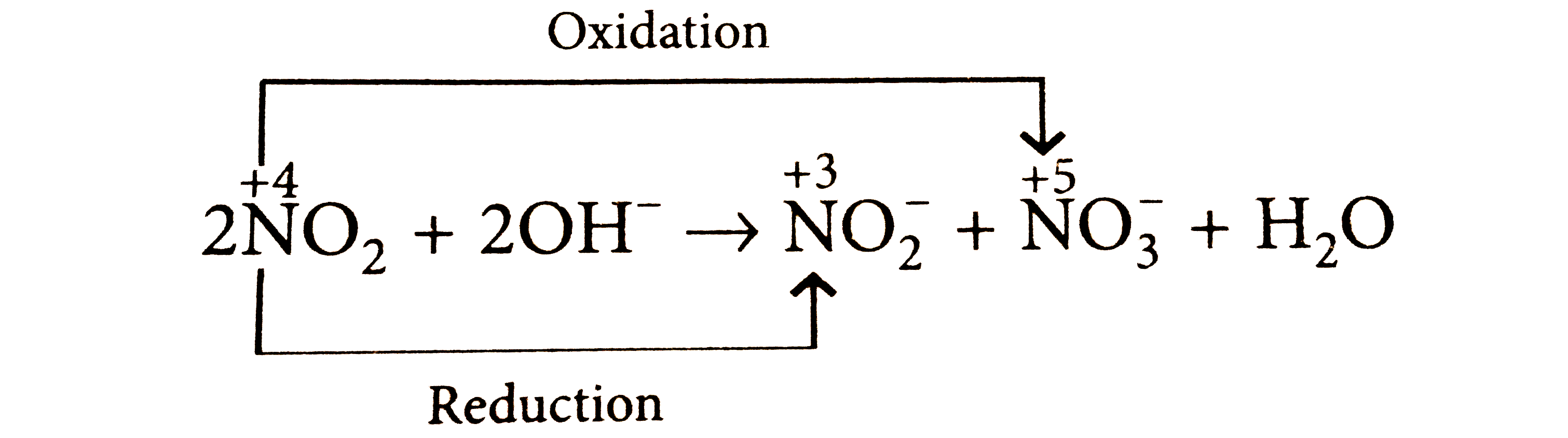
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosREDOX REACTIONS
NCERT FINGERTIPS|Exercise Higher Order Thinking Skills|9 VideosPRACTICE PAPER 3
NCERT FINGERTIPS|Exercise Practice Paper 3|46 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|10 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-REDOX REACTIONS-NCERT Exemplar
- Which of the following is not an example of redox reaction?
Text Solution
|
- The more positive the value of E^(θ), the greater is the tendency of t...
Text Solution
|
- E^(θ) values of some redox couples are given below. On the basis of th...
Text Solution
|
- Using the standard electrode potential, find out the pair between whic...
Text Solution
|
- Thiosulphate reacts differently with iodine and bromine in the reactio...
Text Solution
|
- The oxidation number of an element in a compound is evaluated on the b...
Text Solution
|
- In which of the following compounds, an elements exhibits two differen...
Text Solution
|
- Which of the following arrangements represent increaseing oxidation nu...
Text Solution
|
- The largest oxidation number exhibited by an element depends on its ou...
Text Solution
|
- Identify the disproportionation reaction.
Text Solution
|
- Which of the following elements does not show disproportionation tende...
Text Solution
|