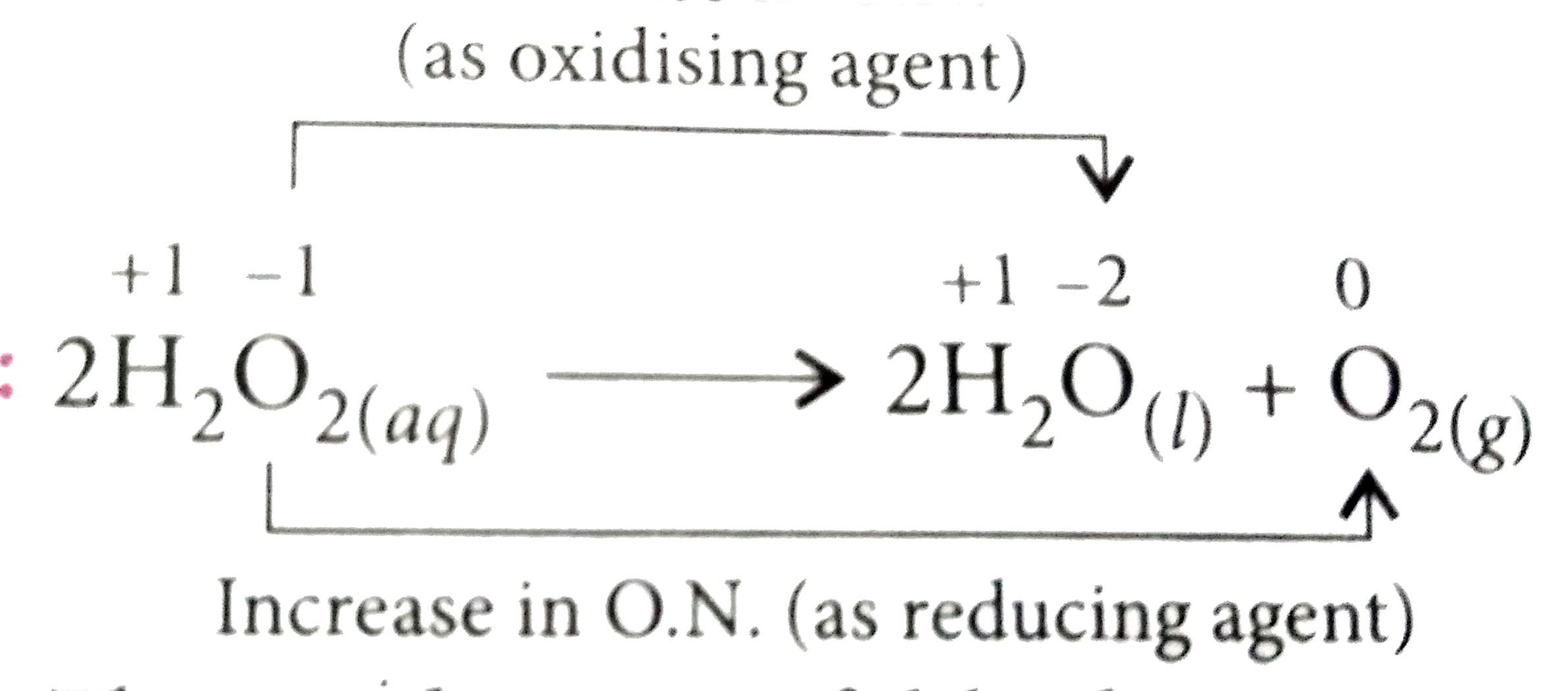A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROGEN
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosHYDROGEN
NCERT FINGERTIPS|Exercise Higher Order Thinking Skills|9 VideosHYDROCARBONS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT FINGERTIPS|Exercise Assertion And Reason|12 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-HYDROGEN -NCERT Exemplar
- Hydrogen resembles halogens in many respects for which several fac...
Text Solution
|
- Why does H^(+) ion always get associated with atoms or molecules ...
Text Solution
|
- Metal hydrides are ionic, covalent or molecular in nature. Among LiH, ...
Text Solution
|
- Which of the following hydrides is electron-precise hydride ?
Text Solution
|
- Radioactive elements emit alpha, beta and gamma rays and are character...
Text Solution
|
- Cosider the reactions (i) H(2)O(2) + 2HI to I(2) + 2H(2)O (ii...
Text Solution
|
- The oxide that give H(2)O(2) on treatment with dilute H(2)SO(4) is
Text Solution
|
- Which of the following equations depict theoxidising nature of H(2)O(2...
Text Solution
|
- Which of the following equation depicts reducing nature of H(2)O(2)...
Text Solution
|
- Hydrogen peroxide is
Text Solution
|
- Which of the following reaction increases, production of dihydrogen fr...
Text Solution
|
- When sodium peroxide is trated with the dilute sulphuric acid, we...
Text Solution
|
- Hydrogen peroxide is obtained by the electrolysis of .
Text Solution
|
- Which of the following reactiona is an example of use of water gas...
Text Solution
|
- Which of the following ions will cause hardness in water sample?
Text Solution
|
- Which of the following compounds is used for water softening ?
Text Solution
|
- Elements of which of the following group(s) of periodic table do ...
Text Solution
|
- Only one element of forms hydrode.
Text Solution
|