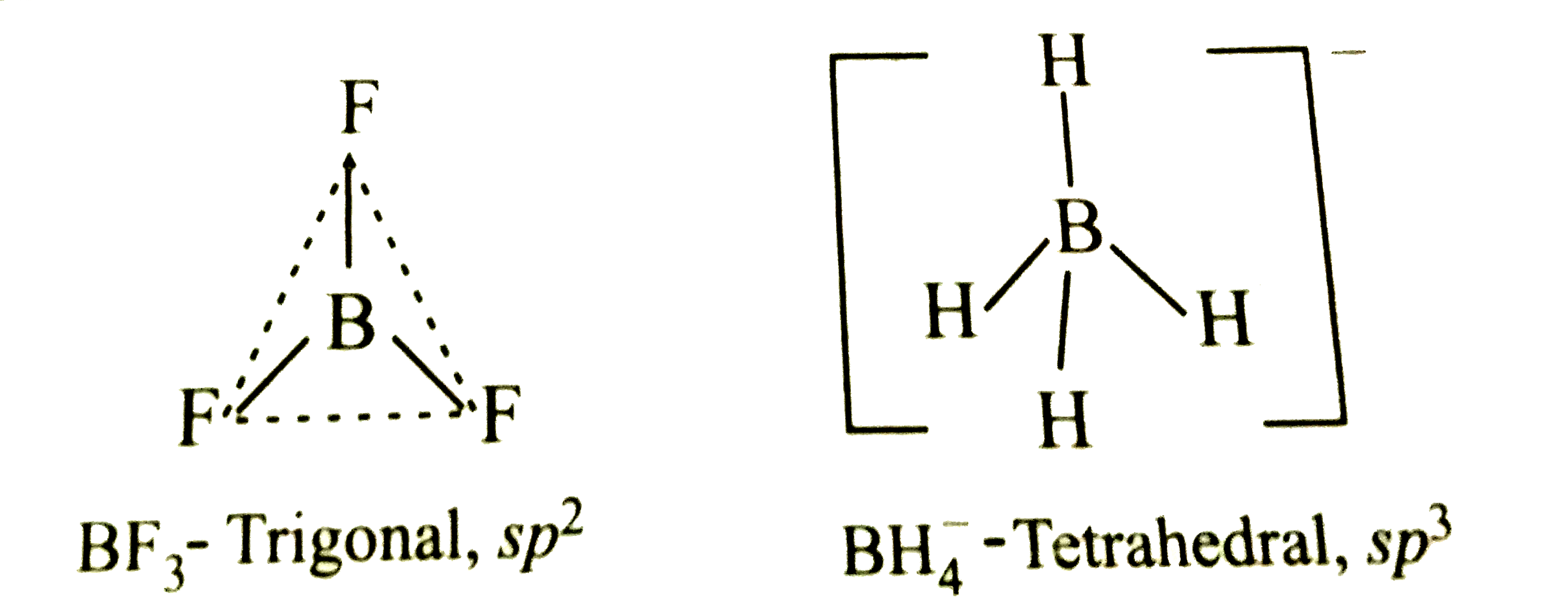A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Important Trends And Anomalous Properties Of Boron|4 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Some Important Compounds Of Boron|20 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosSTRUCTURE OF ATOM
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosTHE S-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THE P-BLOCK ELEMENTS-Group 13 Elements - The Boron Family
- The first ionisation potential of Al is smaller than that of Mg becaus...
Text Solution
|
- The electropositive first increases from B to Al and then decreases fr...
Text Solution
|
- Group 13 elements show
Text Solution
|
- Aluminium exhibits +3 oxidation state. As we move down the group, +1 o...
Text Solution
|
- Group 13 elements show +1 and +3 oxidation states. Relative statibilit...
Text Solution
|
- Which of the following group 13 elements does not show the inert pair ...
Text Solution
|
- Which has the maximum electropositive character ?
Text Solution
|
- Describe the shapes of BF(3) and BH(4)^(-). Assign the hybridisation o...
Text Solution
|
- Halides of boron and aluminium are Lewis Acids. Assign reason.
Text Solution
|
- Which of the following is not true regarding the nature of halides of ...
Text Solution
|
- Which of the following hydroxide is acidic?
Text Solution
|
- The decreasing order of power of boron halides to act as Lewis acids i...
Text Solution
|
- The aqueous solution of AICI(3) is acidic due to
Text Solution
|
- The aqueous solution of AICI(3) is acidic due to
Text Solution
|
- Anhydrous aluminium chloride is prepared by
Text Solution
|
- The reduction of an oxide by aluminium is called
Text Solution
|
- Why does AlCl(3) fumes in air?
Text Solution
|
- Which of the following does not show similarity between boron and alum...
Text Solution
|
- A metal X reacts with aqueous NaOH solution to form Y and a highly inf...
Text Solution
|
- A metal M reacts with sodium hydroxide to give a white precipitate X w...
Text Solution
|