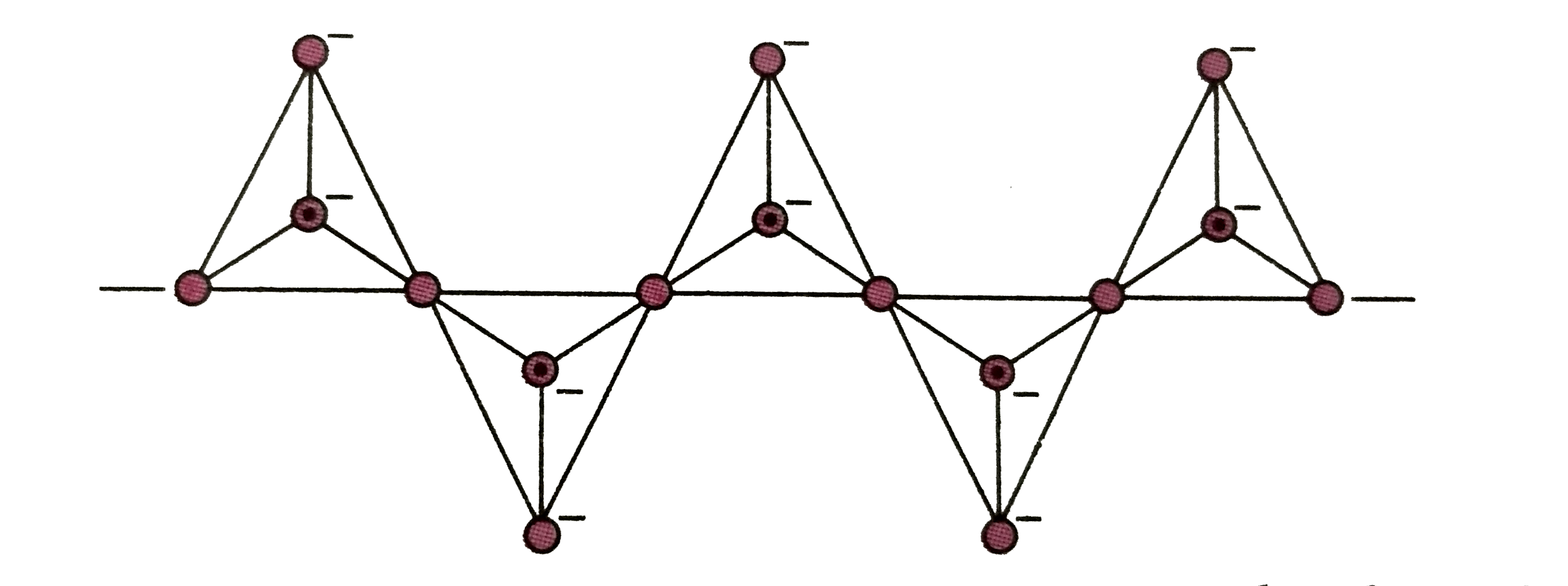
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Higher Order Thinking Skills|9 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise NCERT Exemplar|15 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Allotropes Of Carbon|8 VideosSTRUCTURE OF ATOM
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosTHE S-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THE P-BLOCK ELEMENTS-Some Important Compounds Of Carbon
- which property of CO(2) makes it of biological and geo-chemical improt...
Text Solution
|
- CO(2) is not a poisnous gas but there is increase in concentration of ...
Text Solution
|
- which of the following is not true about structure of carbon dioxide?
Text Solution
|
- Mark the example which is not correct
Text Solution
|
- Silicon is an important constituent of
Text Solution
|
- Complete the following reactions: ? (i) SiO(2) + 2NaOH to X + H(2) O ...
Text Solution
|
- Which of the following is compounds cannot be stored in glass vessels ...
Text Solution
|
- Which of the following properties correctly explain SiO(2)?
Text Solution
|
- SiCl(4) overset(H(2)O)(to) X overset("Heat")(to) Y overset(NaOH)(to) Z...
Text Solution
|
- Which is incorrect statement about silicones ?
Text Solution
|
- which of the following bonds is shown in silicones?
Text Solution
|
- An oxide X in the normal form is almost non-reactive due to very high ...
Text Solution
|
- What happens when: (i). Methyl chloride is treated with alcoholic KC...
Text Solution
|
- [SiO(4)]^(4-) has tetrahedral structure, the silicate formed by using ...
Text Solution
|
- Match the Column I with Column II and mark the approprite choice. {:...
Text Solution
|
- Match the colomn I with column II and mark the appropriate choice. {...
Text Solution
|
- Glass and cement are two important examples of
Text Solution
|
- which of the following is not matched correctly with its use?
Text Solution
|
- A type of zeolite used to convert alcohols directly into gasoline is
Text Solution
|
- Which of the following statements about the zeolites is false?
Text Solution
|