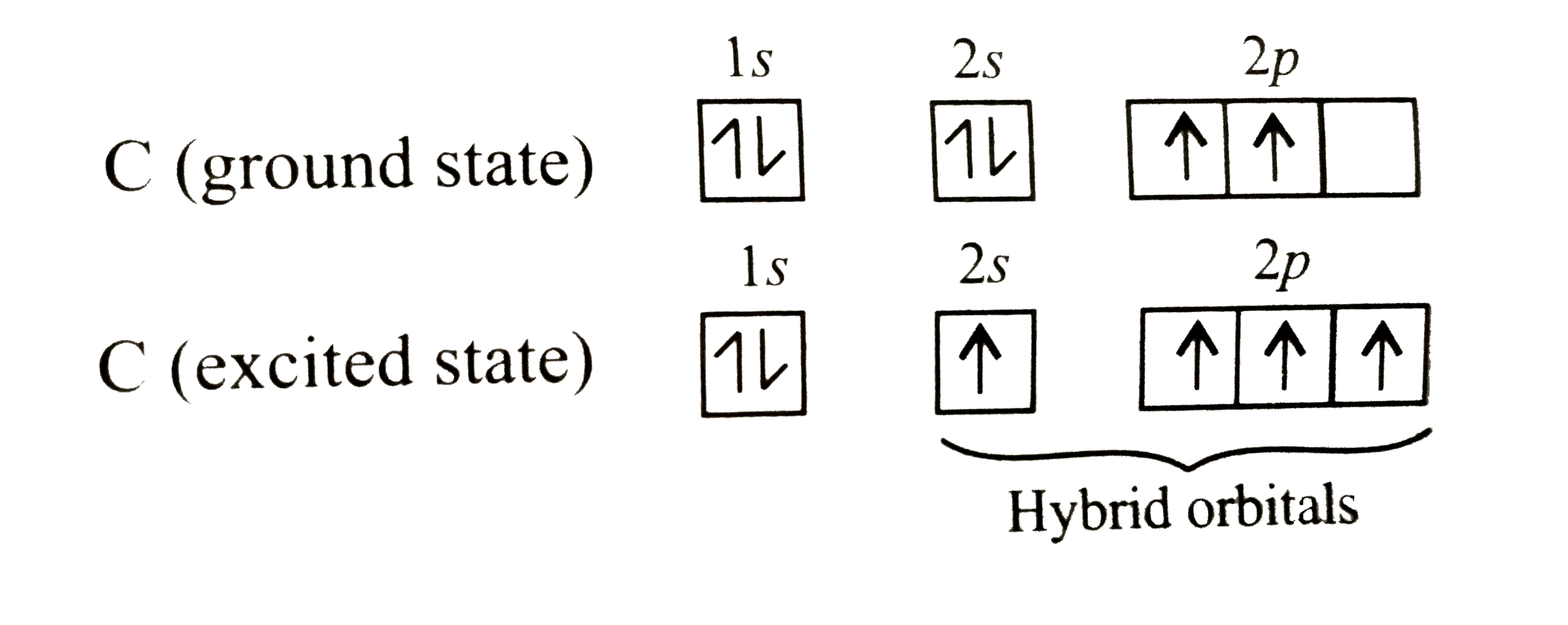Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THE P-BLOCK ELEMENTS-Assertion And Reason
- Assertion : In p-block elements, a lot of variation in properties of e...
Text Solution
|
- Assertion : Compounds formed between non-metals are largely covalent i...
Text Solution
|
- Assertion:- The heavier P-block elements form storng pi- bonds. Rea...
Text Solution
|
- Assertion : Boron forms only covalent compounds. , Reason : Boron ha...
Text Solution
|
- Assertion : Atomic radius of Ga is larger than that of aluminium Rea...
Text Solution
|
- Assertion : Although aluminium is above hydrogen in electrochemical se...
Text Solution
|
- Assertion : In diborane, each B atom is sp^(2) hybridised. Reason : ...
Text Solution
|
- Assertion : Carbon atom is tetravalent though it has two unpaired elec...
Text Solution
|
- Assertion : Sn in +2 oxidation state is a reducing agent while Pb in +...
Text Solution
|
- Assertion : Diamond is the hardest substance on the earth. Reason : ...
Text Solution
|
- Assertion : Fullerenes are the only pure form of carbon. Reason : It...
Text Solution
|
- Assertion : Carbon monoxide is a poisonous gas Reason : Carbon monox...
Text Solution
|
- Assertion : In CO(2) molecule C atoms undergoes sp^(2) hybridisation ...
Text Solution
|
- Assertion : CO(2) is a gas at room temperature while SiO(2) is a cryst...
Text Solution
|
- Assertion : Zeolites are the three-dimensional network silicates. Re...
Text Solution
|