Similar Questions
Explore conceptually related problems
Recommended Questions
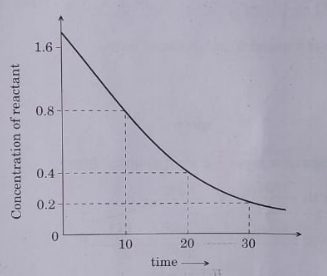
- Analyse the given graph,drawn between concentration of reactant vs. ti...
Text Solution
|
- For a reaction , a graph was plotted between reactant concentration c ...
Text Solution
|
- For a zero order reaction, the plot of concentration of a reactant vs ...
Text Solution
|
- For a zero order reaction, the plot of concentration of a reactant vs ...
Text Solution
|
- Certain reactions follow the relation between concentrations of the re...
Text Solution
|
- Analyse the given graph,drawn between concentration of reactant vs. ti...
Text Solution
|
- Drawn the graph that the concentration 'R', of the reactant and 't' th...
Text Solution
|
- If 'a' is the initial concentration of the reactant, the time taken fo...
Text Solution
|
- What will be the nature of the graph showing the concentration of the ...
Text Solution
|