Similar Questions
Explore conceptually related problems
Recommended Questions
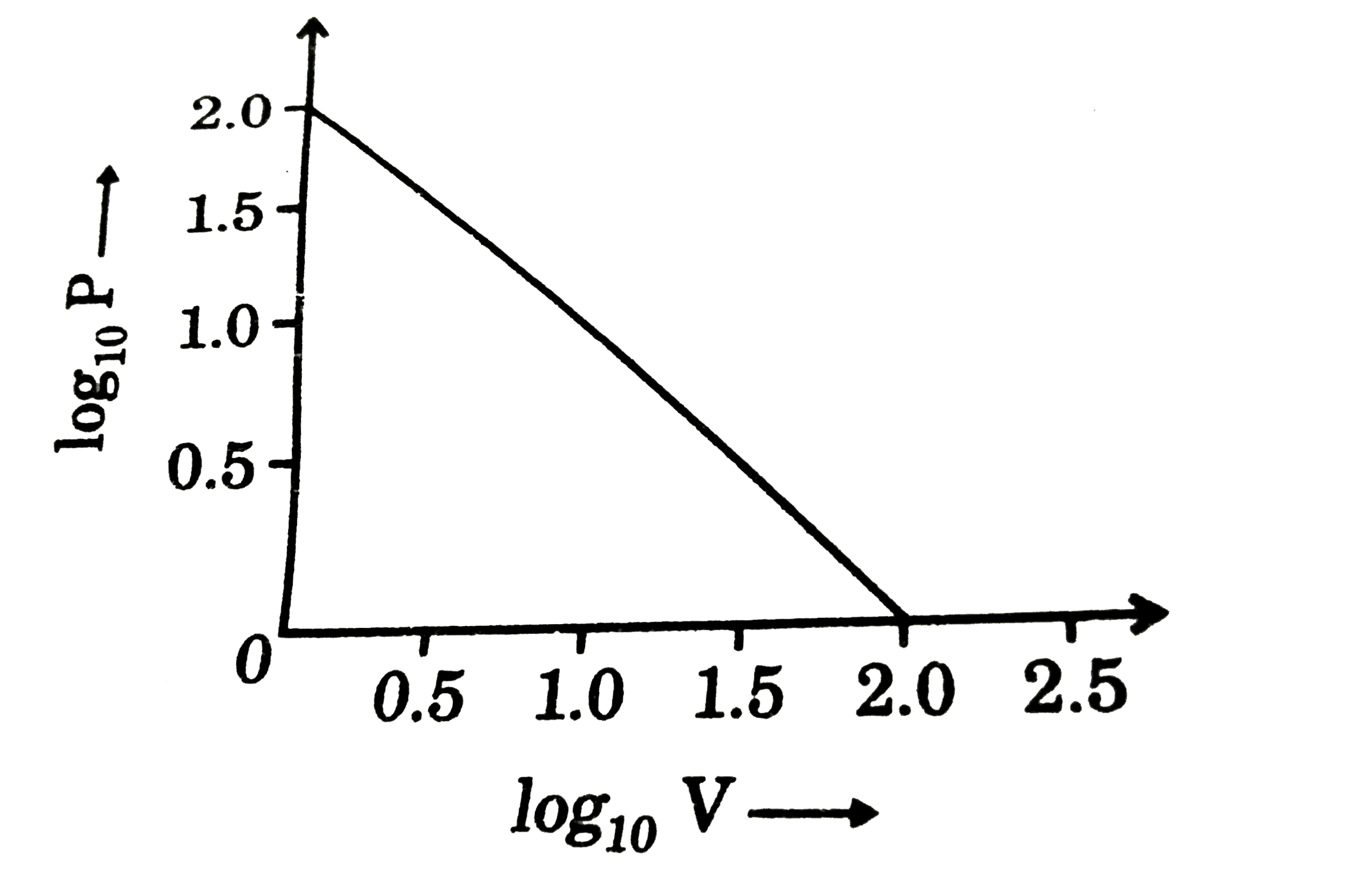
- For the given isotherm (P in atm and V in L) for one mole of an ideal ...
Text Solution
|
- Two moles of an ideal gas undergo the following process : (a) a reve...
Text Solution
|
- Calculate pressure (in atm) of a Vander-waal gas taken at temperature ...
Text Solution
|
- The van der Waal's constants for a gas are a=1.92 atm L^(2) "mol"^(-2)...
Text Solution
|
- One mole of an ideal gas at 27^(@) ,8.21 atm absorbs 420 cal of heat d...
Text Solution
|
- For the given isotherm (P in atm and V in L) for one mole of an ideal ...
Text Solution
|
- One mole of an ideal gas undergoes a process (10L, 4atm) to (4L, 10 at...
Text Solution
|
- At a constant pressure P, the plot of volume (V) as a function of temp...
Text Solution
|
- Vapour density of an ideal gas which has a density 2.16 g//L at 1.12 a...
Text Solution
|