A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BHARATI BHAWAN (ENGLISH)-PRACTICALS -EXERCISE
- The order of rectivity of Zn, Fe, Cu and Al is
Text Solution
|
- A piece of granulated zinc was dropped into copper sulphate solution. ...
Text Solution
|
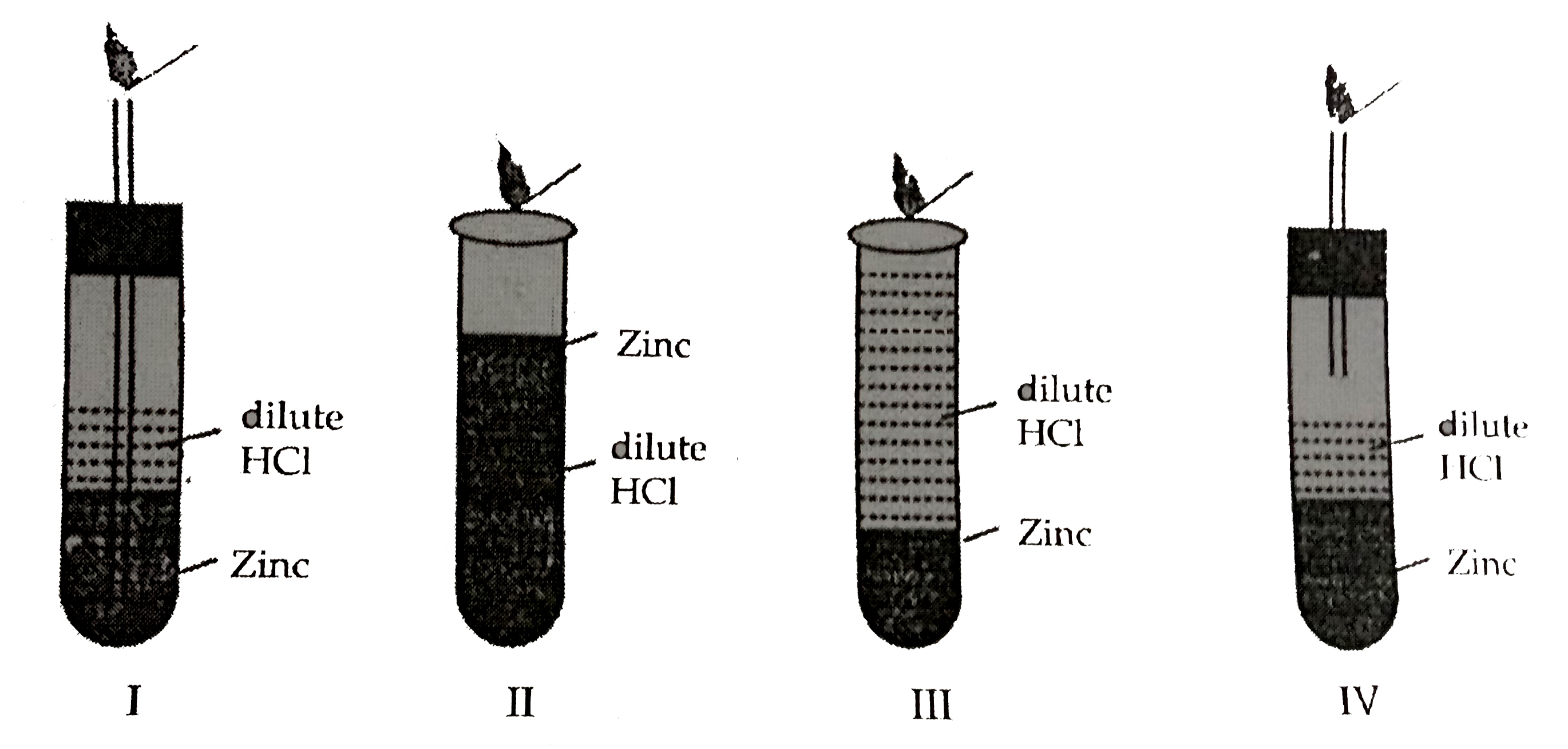
- Four set ups a given below were arranged to identify the gas evolved ...
Text Solution
|
- When an iron rod is dipped into a solution of copper sulphate, copper ...
Text Solution
|
- The reaction BaCl(2) +Na(2)SO(4) to BaSO(4) + 2NaCl is an example of a
Text Solution
|
- The reaction respresented by the eqaution CuSO(4) + Fe to FeSO(4) +...
Text Solution
|
- The burning of magnesium in air is a
Text Solution
|
- Which of the following is a decomposition reaction ?
Text Solution
|
- Which of the following reaction is feasible ?
Text Solution
|
- Which of the following reactions is possible ?
Text Solution
|
- The general formula of carbodxylic acids is
Text Solution
|
- Acetic acid is essentially present in
Text Solution
|
- Acetic acid is
Text Solution
|
- The IUPAC name of acetic acid is
Text Solution
|
- Acetic acid is
Text Solution
|
- When a piece of sodium metal is dipped into acetic acid, a colourless,...
Text Solution
|
- Some vinegar is dropped on solid sodium carbonate. Brisk efferevescenc...
Text Solution
|
- 5 mL of acetic acid is dissolved in 20mL of water. The volume of the s...
Text Solution
|
- When a blue litmus paper is dropped into a dilute solution of acetic a...
Text Solution
|
- The product formed when ethyl alcohol is heated with acetic acid in pr...
Text Solution
|