A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTRONS AND PROTONS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CORNER|19 VideosELECTRONS AND PROTONS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise Exercise 1|50 VideosELECTROMAGNETIC INDUCTION
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CONER|34 VideosELECTROSTATICS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|29 Videos
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-ELECTRONS AND PROTONS-Exercise 2
- The act of photoelectric effect taking place with a certain photosensi...
Text Solution
|
- A point source of light is used in a photoelectric effect. If the sour...
Text Solution
|
- If the frequency of light in a photoelectric experiment is doubled the...
Text Solution
|
- The cathode of a photoelectric cell is changed such that the work func...
Text Solution
|
- In a photoelectric experiment, the potential difference V that must be...
Text Solution
|
- what should be the velocity of an electron so that its momentum becom...
Text Solution
|
- From the figure describing photoelectric effect we may infer correctly...
Text Solution
|
- Cathode rays enter a magnetic field makin oblique angle withh the line...
Text Solution
|
- The work function for aluminium is 4.125 eV. The cut-off wavelength fo...
Text Solution
|
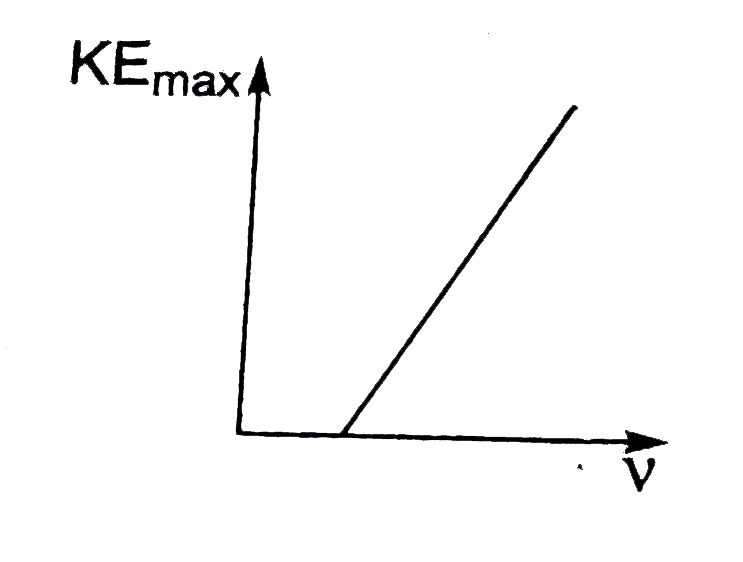
- According to Einstein's photoelectric equation, the plot of the maximu...
Text Solution
|
- Photon and electron are given same energy (10^(-20) J). Wavelength ass...
Text Solution
|
- Match column I (fundamental experiment) with column II (its conclusion...
Text Solution
|
- When a certain metallic surface is illuminated with monochromatic ligh...
Text Solution
|
- Which of the following figure represents the variation of particle mom...
Text Solution
|
- Threshold wavelength for lithium metal is 6250 Å. For photo emission, ...
Text Solution
|
- In a photoelectric experiment the relation between applied potential d...
Text Solution
|
- Photoelectric effect experiments are performed using three different m...
Text Solution
|
- Light of two different frequencies whose photons have energies 1eV and...
Text Solution
|
- Light of intensity 10^(-5)Wm^(-2) falls on a sodium photocell of surfa...
Text Solution
|
- Work function for caesium metal is 2.14 eV. Let a beam of light of fre...
Text Solution
|
 .
.