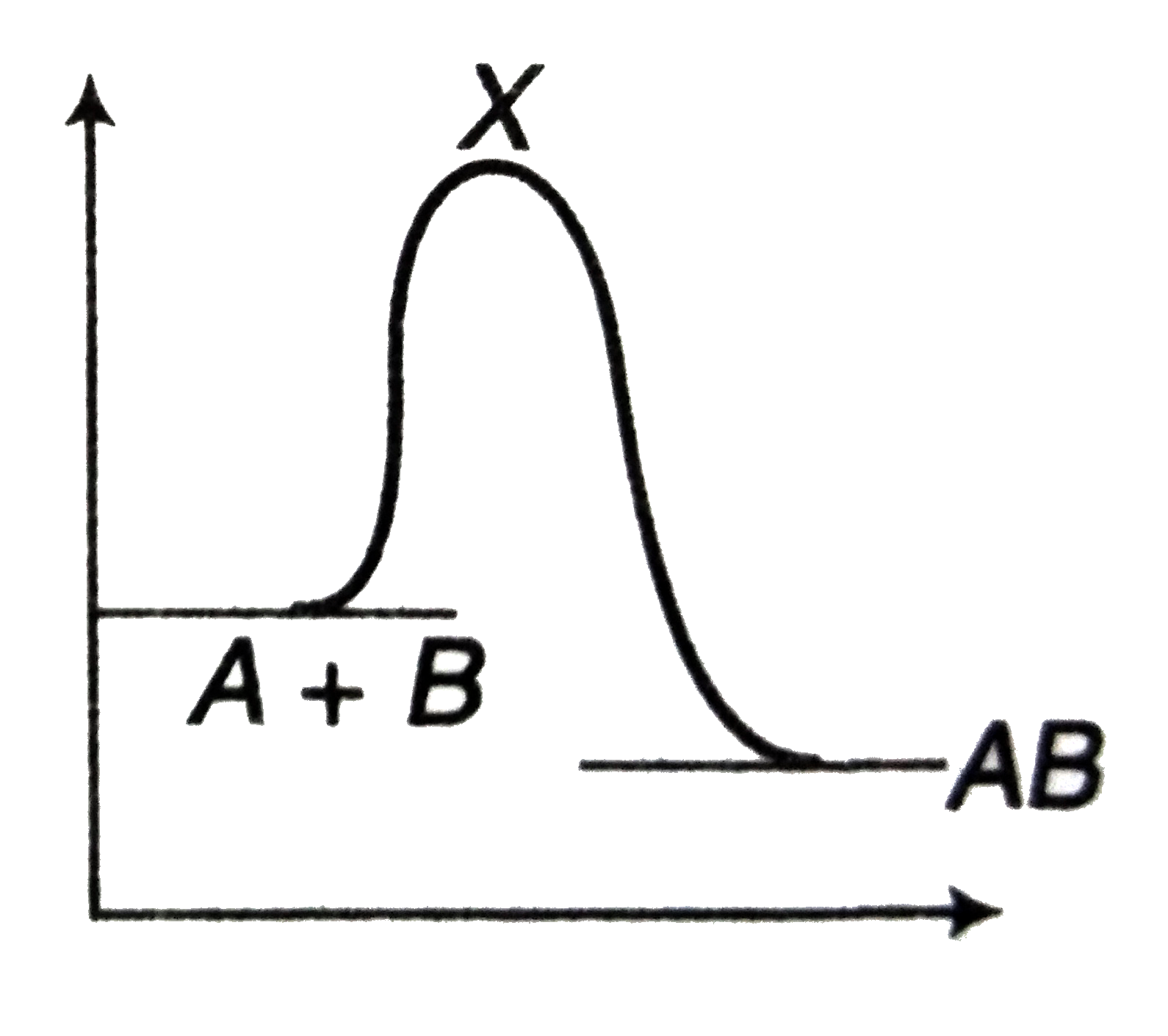
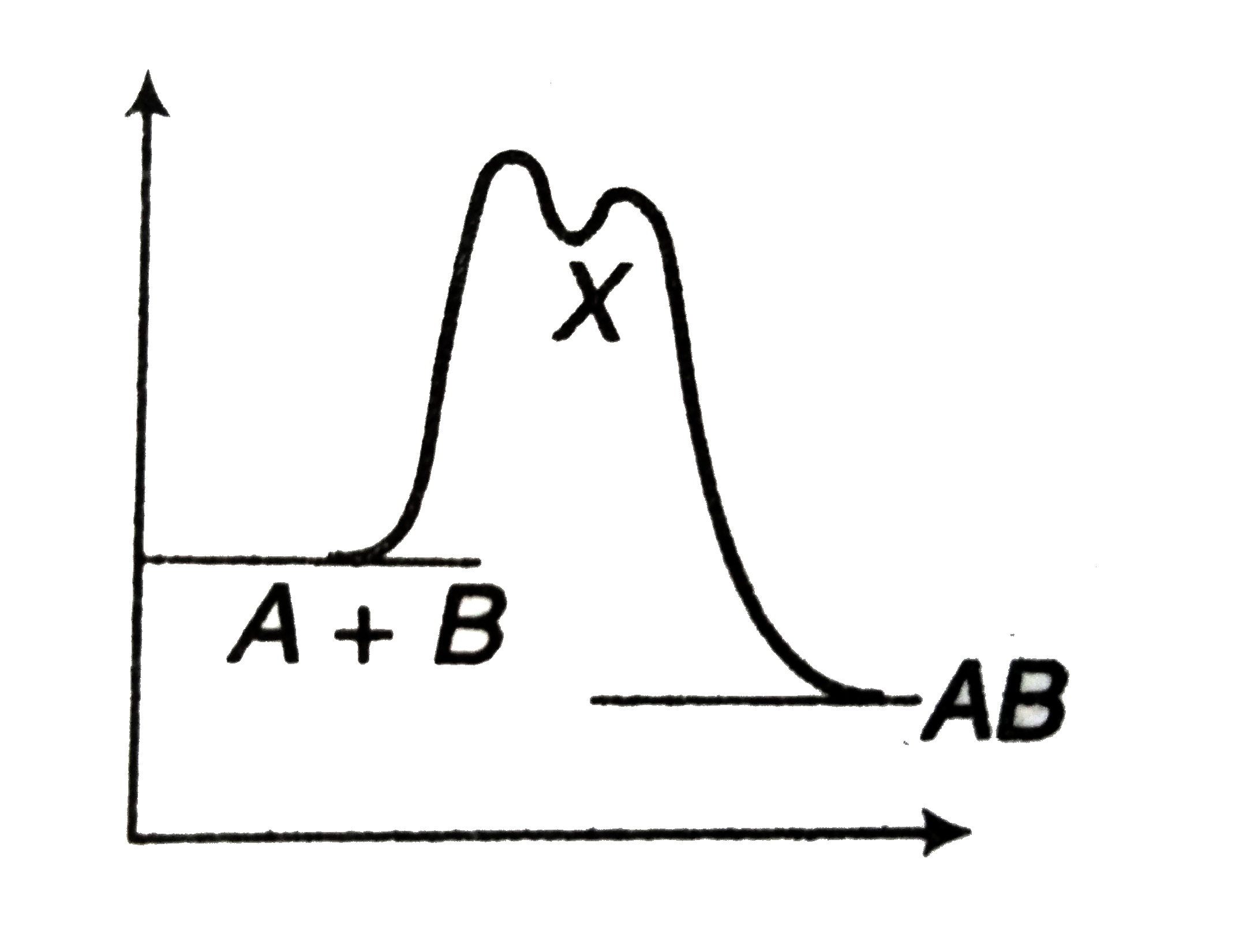
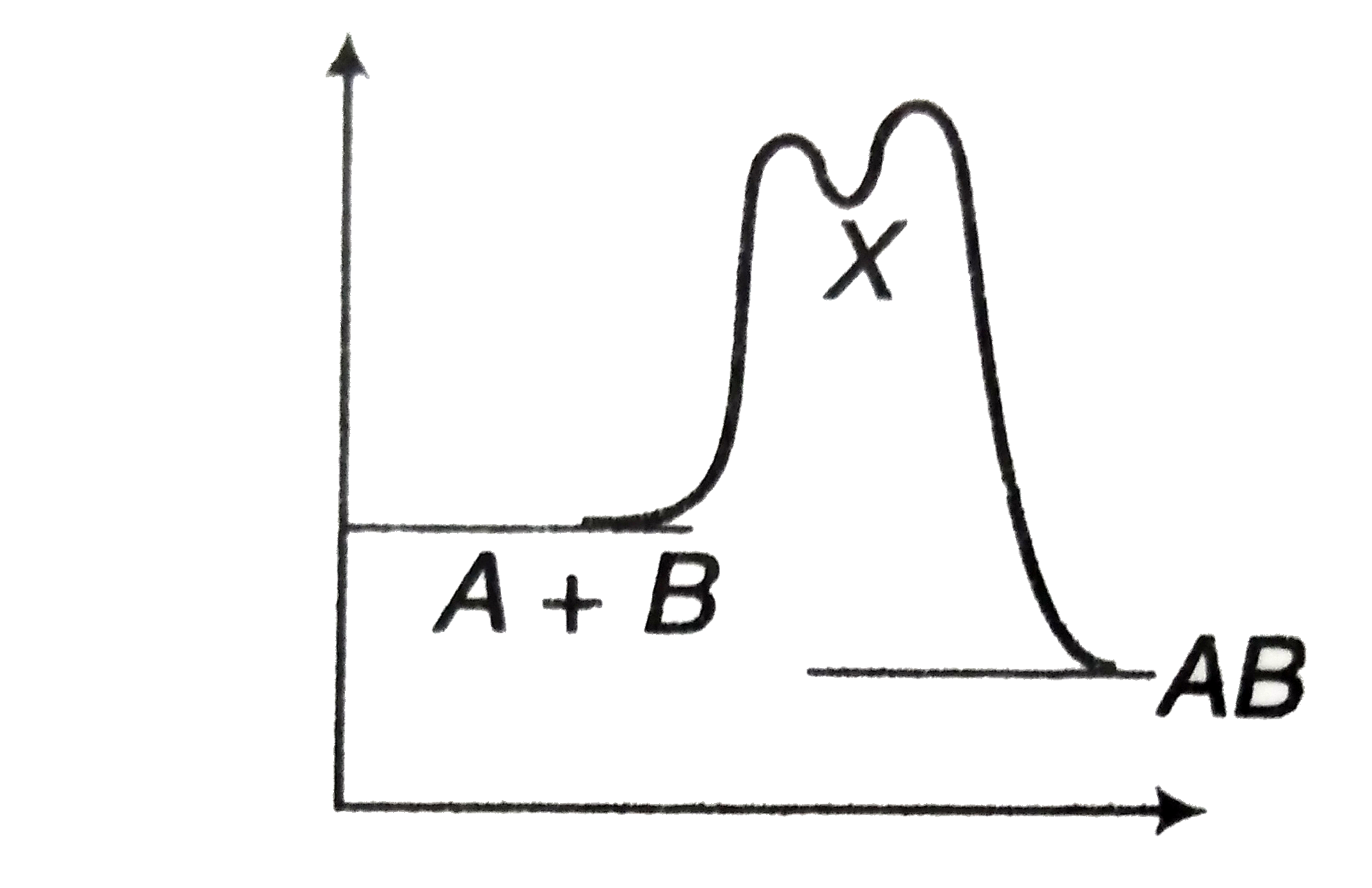
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CORNER|20 VideosCHEMICAL KINETICS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise Exercise 1|63 VideosBIOMOLECULES
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CORNER|24 VideosCHEMICAL THERMODYNAMICS AND ENERGETIC
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|40 Videos
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-CHEMICAL KINETICS -Exercise 2
- In the sequence of reaction, L overset(k(1))rarr M overset(k(2))rar...
Text Solution
|
- Half-life of a reaction is found to be inversely proportional to the c...
Text Solution
|
- For an exothermic chemical process occurring in two as (i) A + B rar...
Text Solution
|
- A reactant (A) forms two products A overset (k(1))rarr B, Activation...
Text Solution
|
- In a first order reaction, ArarrP, the ratio of a//(a-x) was found to...
Text Solution
|
- The rate of a chemical reaction becomes double for every 10^(@) rise i...
Text Solution
|
- For a reaction 1//2A to 2B, rate of disappearance of A is related to t...
Text Solution
|
- The rate of a reaction doubles when its temperature changes form 300 K...
Text Solution
|
- A plot of "Ink "v//s " 1/T" for a reaction gives the slope -1xx10^(4)k...
Text Solution
|
- In a zero-order reaction for every 10^(@) rise of temperature, the rat...
Text Solution
|
- During the kinetic study of the reaction 2A+BrarrC+D, following result...
Text Solution
|
- The inversion of cane sugar is first order in [sugar] and proceeds wit...
Text Solution
|
- The initial rates of reaction 3 A+ 2B +C rarr products at different in...
Text Solution
|
- For a reaction , 2N(2)O(5)rarr4NO(2)+O(2), the rate is directly propo...
Text Solution
|
- At 500 K, the half-life period of a gaseous reaction at the initial pr...
Text Solution
|
- The energies of activation for forward and reverse reaction for A(2)+...
Text Solution
|
- A reaction was found to be second order with respect to the concentrat...
Text Solution
|
- For the second order reaction, A+Brarr"products" when a moles of A...
Text Solution
|
- A(g)overset(Delta)rarrP(g)+Q(g)+R(g), follows first order kinetics wit...
Text Solution
|
- The value of rate constant for a first order reaction is 2.30...
Text Solution
|