Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS|16 VideosSOLID STATE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|8 VideosSOLID STATE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|27 VideosPOLYMERS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|6 VideosSOLUTIONS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|10 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-SOLID STATE-VERY SHORT ANSWER QUESTIONS
- What is meant by the term "coordination number"? b. What is the coor...
Text Solution
|
- What is the co-ordination number of each sphere in cubic close packing...
Text Solution
|
- The coordination number of each atom in body centered cubic unit cell...
Text Solution
|
- a. "Stability of a crystal is reflected is reflected in the magnitude ...
Text Solution
|
- How are the intermolecular forces among the molecules affect the melti...
Text Solution
|
- How do you distinguish between hexagonal close - packing and cubic clo...
Text Solution
|
- How do you distinguish between crystal lattice and unit cell ?
Text Solution
|
- How many lattice points are there in one unit cell of face centered cu...
Text Solution
|
- How many lattice points are there in one unit cell of each of the foll...
Text Solution
|
- How many lattice points are there in one unit cell of body centered cu...
Text Solution
|
- What is a semi conductor ?
Text Solution
|
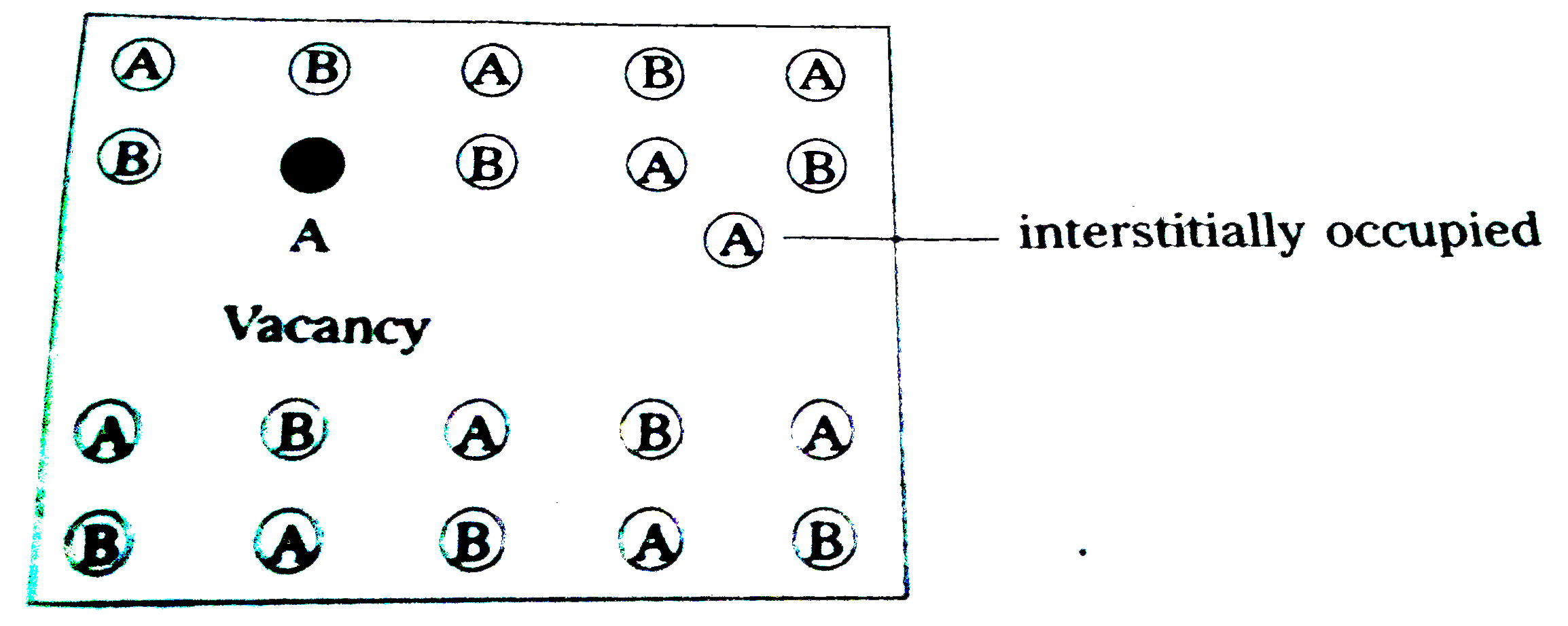
- What is Schottky defect ?
Text Solution
|
- What is Frenkl defect ?
Text Solution
|
- What is interstitial defect ?
Text Solution
|
- What are f-centers ?
Text Solution
|
- Explain Ferromagnetism with suitable example .
Text Solution
|
- Explain paramagnetism with suitable example .
Text Solution
|
- Explain Ferrimagnetisms with suitable example.
Text Solution
|
- Explain Antiferromagnetism with suitable example .
Text Solution
|
- Why X- rays are needed to probe the crystal structure ?
Text Solution
|