Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|8 VideosSOLID STATE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|27 VideosSOLID STATE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|23 VideosPOLYMERS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|6 VideosSOLUTIONS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|10 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-SOLID STATE-SHORT ANSWER QUESTIONS
- Explain similarities between metallic and ionic crystals .
Text Solution
|
- Explain differences between metallic and ionic crystals .
Text Solution
|
- Explain why ionic solids are hard and brittle .
Text Solution
|
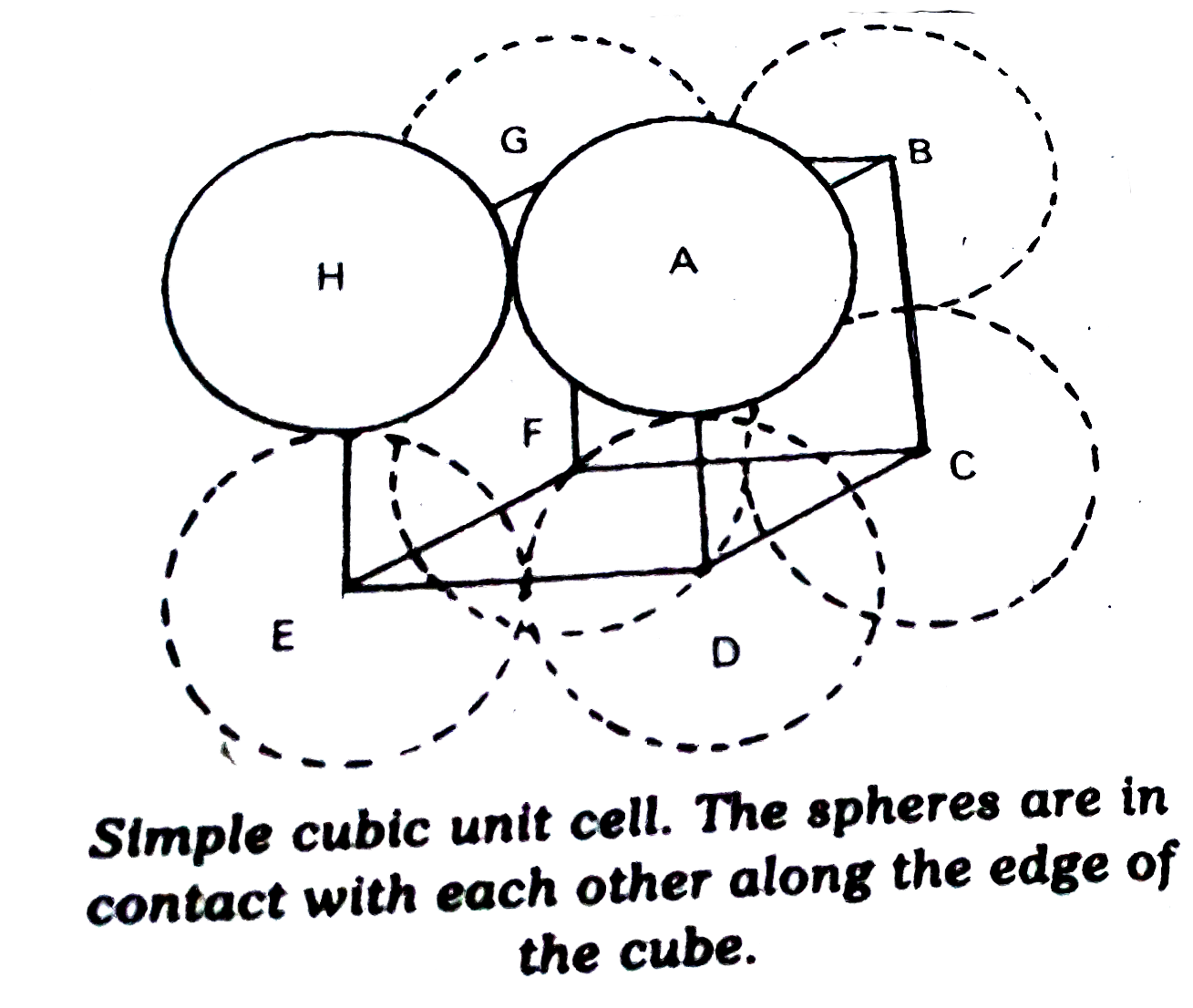
- Calculate the efficiency of packing in case of a metal of simple cubic...
Text Solution
|
- Calculate the efficiency of packing in case of a metal of body centere...
Text Solution
|
- Calculate the efficiency of the packaing in case of face - centered cu...
Text Solution
|
- A cubic solid is made of two elements P and Q . Atoms of Q are at the ...
Text Solution
|
- If the radius of the octahedral void is r and radius of the atoms in c...
Text Solution
|
- Describe the two main types of semiconductors and contrast their condu...
Text Solution
|
- Classify each of the following as either a p-type or a n -type semico...
Text Solution
|
- Analysis shows that nickel oxide has the formula Ni^(0.98) 0 ,1.00 , w...
Text Solution
|
- Gold (atomic radius = 0.144 nm) crystallizes in a face centered unit c...
Text Solution
|
- In terms of band theory , what is the difference between a conductor a...
Text Solution
|
- In terms of band theory , what is the difference between a conductor a...
Text Solution
|
- In NaCl is doped with 1 xx 10^(-3) mol percent of SrCl(2) , what is th...
Text Solution
|
- Derive Bragg's equation .
Text Solution
|