Text Solution
Verified by Experts
Topper's Solved these Questions
SURFACE CHEMISTRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTION|13 VideosSURFACE CHEMISTRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|8 VideosSURFACE CHEMISTRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|8 VideosSOLUTIONS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|10 VideosTELANGANA MARCH-2019
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SECTION C|6 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-SURFACE CHEMISTRY-SHORT ANSWER QUESTIONS
- Name any six enzyme catalysed reaction.
Text Solution
|
- What do you mean by activity and selectivity of catalyst ?
Text Solution
|
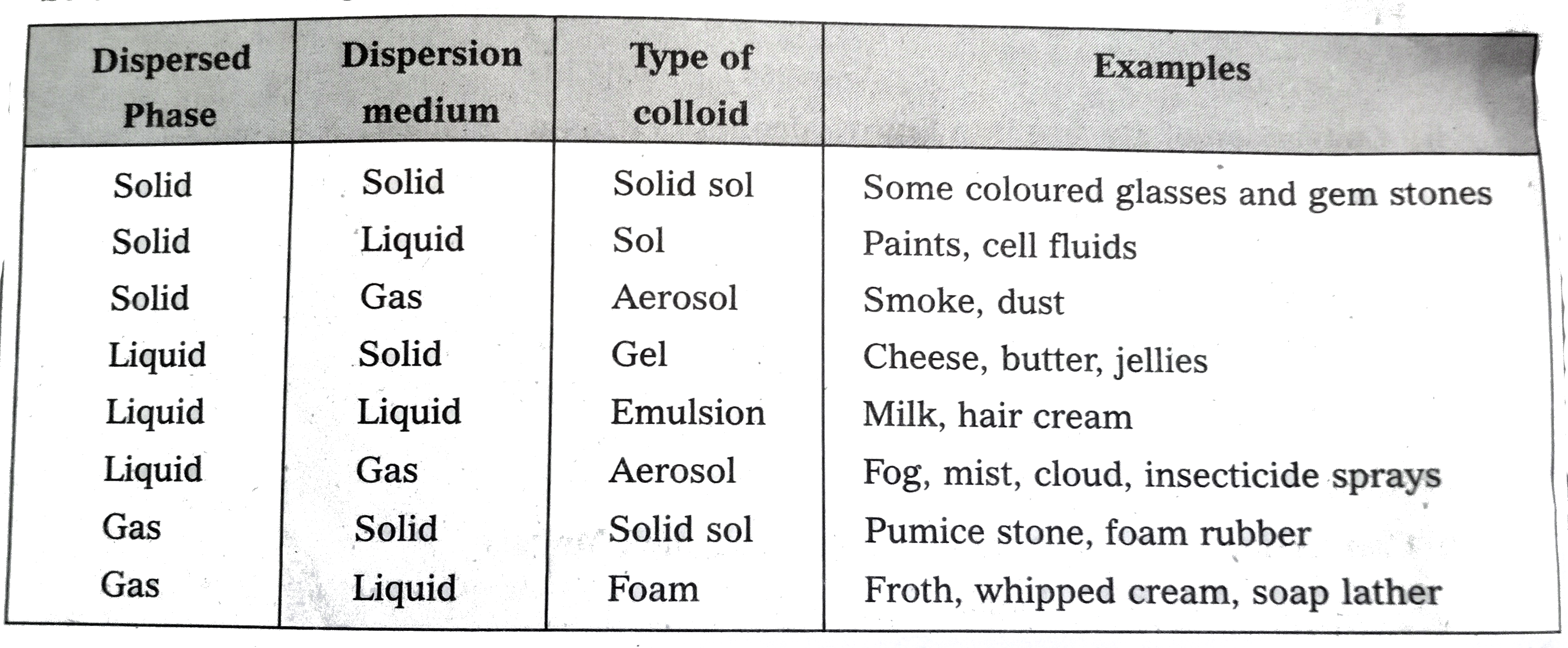
- How are colloids classified on the basis of physical states of compone...
Text Solution
|
- How are colloids classified on the basis of nature of the dispersion ...
Text Solution
|
- How are colloids classified on the basis of interaction between dispe...
Text Solution
|
- What is the difference between a colloidal sol, get, emulsion and a fo...
Text Solution
|
- What are lyophilic and lyophobic sols ? Give one example for each type...
Text Solution
|
- Name a substance whose molecules consist of lyophilic as well as lyoph...
Text Solution
|
- Describe Bredig's arc method of preparation of colloids with a neat di...
Text Solution
|
- Name any four examples of preparation of colloids by chemical methods ...
Text Solution
|
- Describe the purification of colloidal solution by the phenomenon of d...
Text Solution
|
- Explain the formation of micelles with a neat sketch.
Text Solution
|
- Action of soap is due to emulsification and micelle formation. Comment...
Text Solution
|
- Explain the phenomenon of Brownian movement giving reasons for the occ...
Text Solution
|
- Name the four positively charged sols.
Text Solution
|
- Name the four negatively charged sols.
Text Solution
|
- Explain the terms helmholtz electrical double layer and zeta potential...
Text Solution
|
- Explain with a neat sketch the phenomenon of electrophoresis.
Text Solution
|
- What is electrophoresis ?
Text Solution
|
- Explain the Coagulation.
Text Solution
|