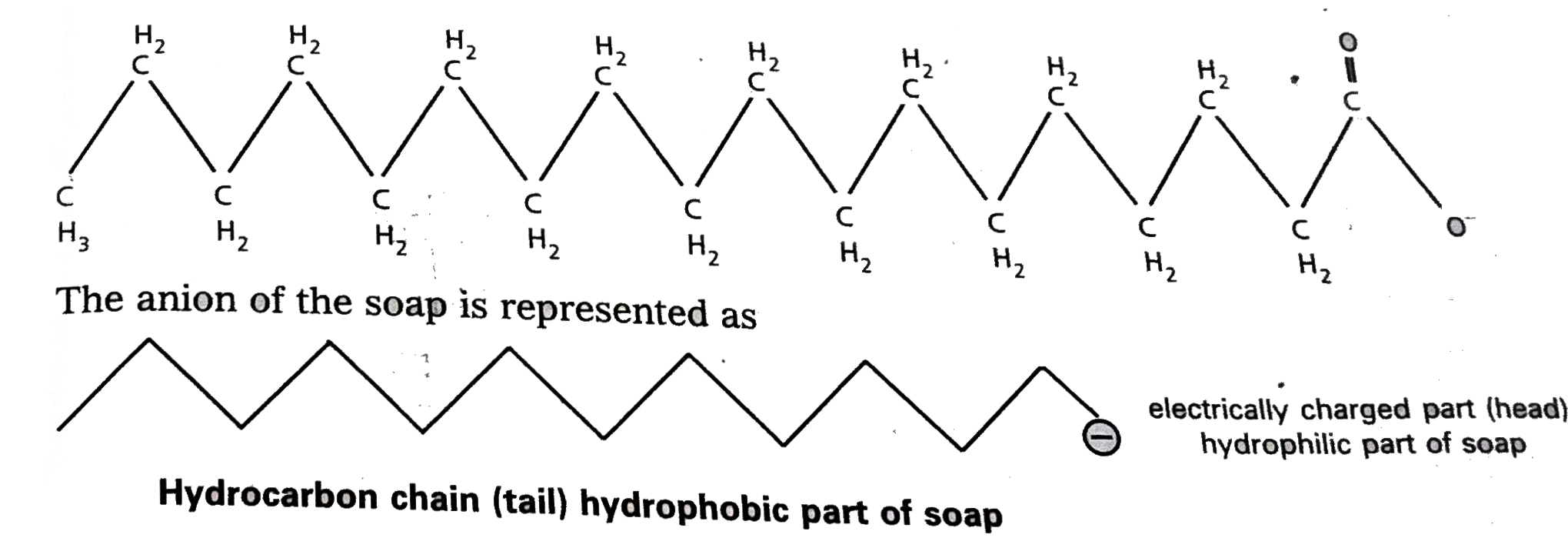
Text Solution
Verified by Experts
Topper's Solved these Questions
SURFACE CHEMISTRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTION|13 VideosSURFACE CHEMISTRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|8 VideosSURFACE CHEMISTRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|8 VideosSOLUTIONS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|10 VideosTELANGANA MARCH-2019
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SECTION C|6 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-SURFACE CHEMISTRY-SHORT ANSWER QUESTIONS
- Describe the purification of colloidal solution by the phenomenon of d...
Text Solution
|
- Explain the formation of micelles with a neat sketch.
Text Solution
|
- Action of soap is due to emulsification and micelle formation. Comment...
Text Solution
|
- Explain the phenomenon of Brownian movement giving reasons for the occ...
Text Solution
|
- Name the four positively charged sols.
Text Solution
|
- Name the four negatively charged sols.
Text Solution
|
- Explain the terms helmholtz electrical double layer and zeta potential...
Text Solution
|
- Explain with a neat sketch the phenomenon of electrophoresis.
Text Solution
|
- What is electrophoresis ?
Text Solution
|
- Explain the Coagulation.
Text Solution
|
- What is dall effect ?
Text Solution
|
- Explain the phenomenon observed When a beam of light is passed thro...
Text Solution
|
- Explain the phenomenon observed An electrolyte, NaCI is added to hyd...
Text Solution
|
- Explain the phenomenon observed An eleetrlc current is passed thro...
Text Solution
|
- Describe cottrell smoke precipitator with a neat diagram.
Text Solution
|
- Amoung NaCl, Na(2)SO(4) , Na(3)PO(4) electrolytes , Na(3)PO(4) which i...
Text Solution
|
- Discuss how a lyophilic colloids protect a lyophobic colloids.
Text Solution
|
- Discuss the use of colloids in Purification of drinking water .
Text Solution
|
- Discuss the use of colloids in Tanning.
Text Solution
|
- Discuss the use of colloids in Medicines.
Text Solution
|