Text Solution
Verified by Experts
Topper's Solved these Questions
GENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|5 VideosGENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|4 VideosGENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|25 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE|18 VideosHALOALKANES AND HALOARENES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SAQ|9 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-GENERAL PRINCIPLES OF MMETALLURGY-SHORT ANSWER QUESTIONS
- Copper can be extracted by hydrometallurgy but not Zinc -explain.
Text Solution
|
- Why is the extraction of copper form pyries more difficult then that f...
Text Solution
|
- Explain Zone refining.
Text Solution
|
- Write down the chemical reactions taking place in the extraction of zi...
Text Solution
|
- Write down the chemical reactions taking place in different zones in t...
Text Solution
|
- How is alumina separated from silica in the bauxite ore associated wit...
Text Solution
|
- Give examples to differentiate roasting and calcination.
Text Solution
|
- The Value of DeltaG^(@) for the formation of Cr(2)O(3) is -"540KJ mol...
Text Solution
|
- What is the role of graphite rod in the electromellurgy of aluminium?
Text Solution
|
- Outline the principles of refining of metals by the following methods....
Text Solution
|
- Predict conditions under which Al might be expected to reduce MgO.
Text Solution
|
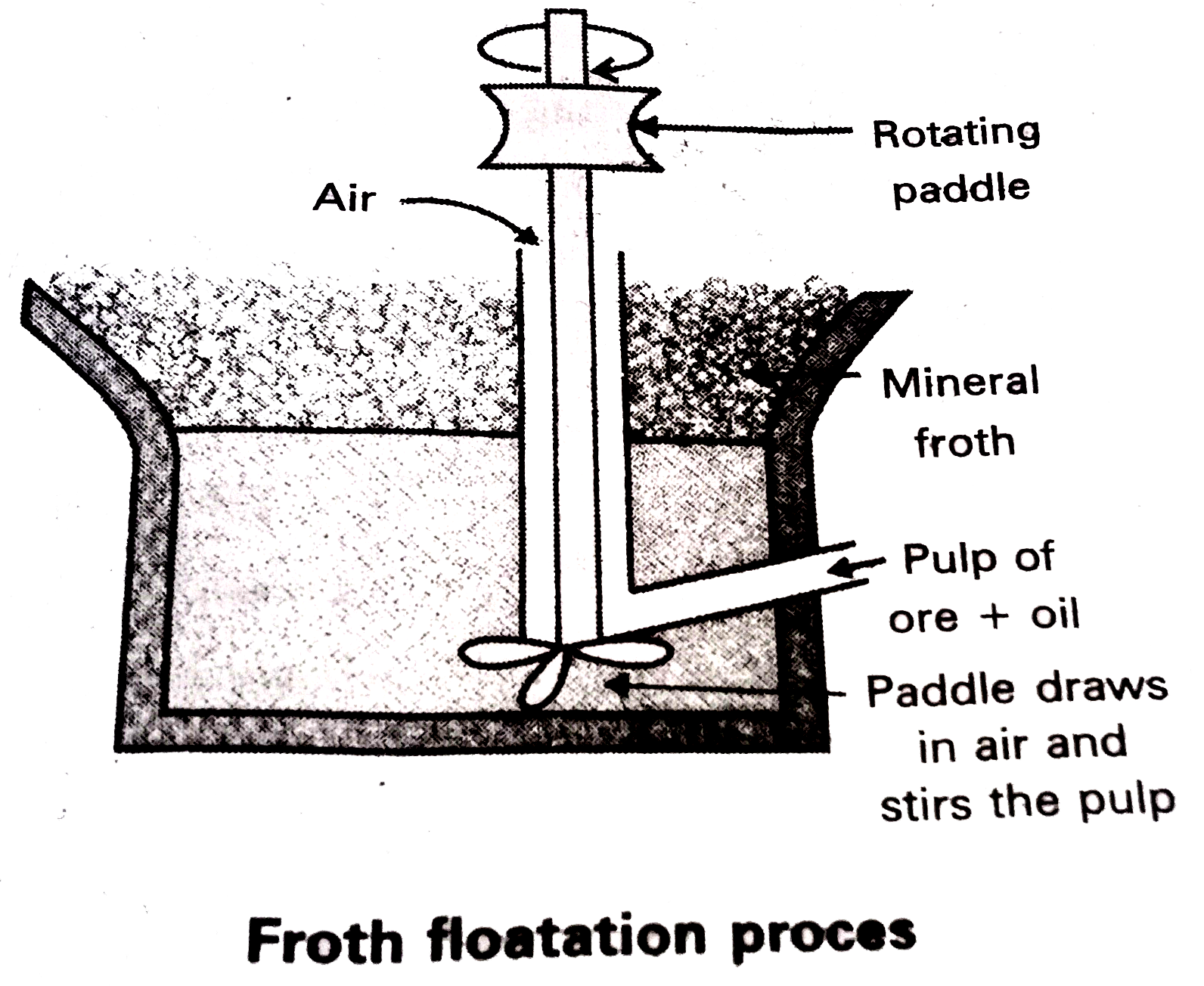
- Explain the purification of sulphide ore by Froth Floatation Method.
Text Solution
|
- Explain the process of leaching of alumina from bauxite.
Text Solution
|
- What is Ellingham diagharm ? What information can be known from this i...
Text Solution
|
- How is copper extracted from copper pyrites ?
Text Solution
|
- Explain the extraction of Zinc form Zinc blende.
Text Solution
|
- Explain smelting process in the extraction of copper.
Text Solution
|
- Explain electrometallurgy with an example.
Text Solution
|
- Explain the process of leaching of alumina from bauxite.
Text Solution
|