Text Solution
Verified by Experts
Topper's Solved these Questions
D AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|71 VideosD AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS|57 VideosCHEMISTRY IN EVERYDAY LIFE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Intex questions|5 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE|18 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-D AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS -INTEXT QUESTIONS
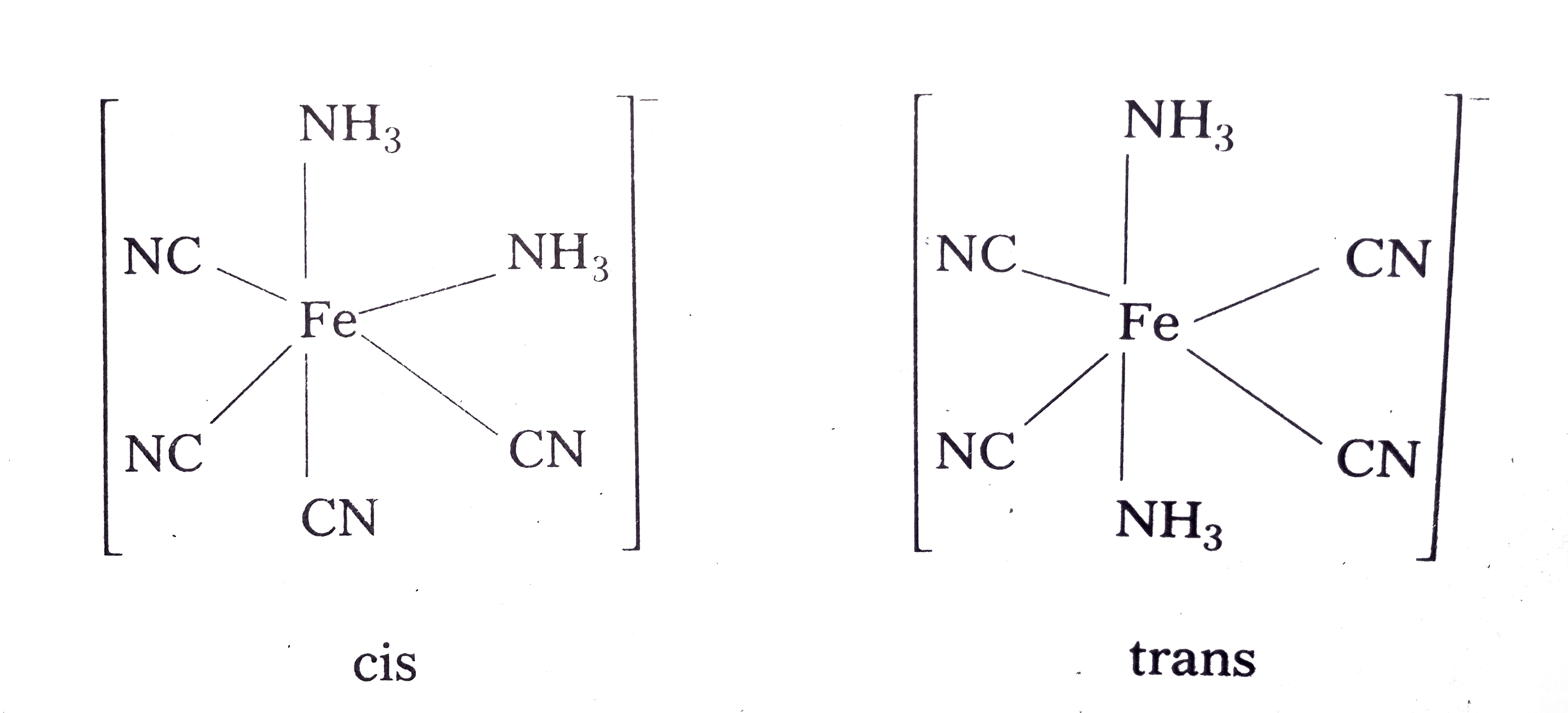
- Draw structures of geometrical isomers of [Fe(NH(3))(2)(CN)(4)]^(-)
Text Solution
|
- Silver atom has completely filled d-orbitals (4d^(10)) in its ground s...
Text Solution
|
- In the series Sc(Z = 21) to Zn (Z = 30), the enthalpy of atomisation o...
Text Solution
|
- Which of the 3d series of the transition metals exhibits the largest n...
Text Solution
|
- The E^(Theta) (M^(2+)//M) value for copper is positive (+0.34V). What ...
Text Solution
|
- How would you account for the irregular variation of ionisation enthal...
Text Solution
|
- Why is the highest oxidation state of a metal exhibited in its oxide o...
Text Solution
|
- Which is a stronger reducing agent Cr^(2+) or Fe^(2+) and why ?
Text Solution
|
- Calculate the 'spin only' magnetic moment of M^(2+)(aq) ion (Z = 27).
Text Solution
|
- Explain why cu^(+) ion is not stable in aqueous solutions?
Text Solution
|
- Actinoid contraction is greater from element to element than lanthanoi...
Text Solution
|
- Write the formulas for the follow co-ordination compounds Tetraammin...
Text Solution
|
- Write the formulae for the follow Co-ordination compounds Potassium ...
Text Solution
|
- Write the formulae for the follow Co-ordination compounds Tris (etha...
Text Solution
|
- Write the formulae for the follow Co-ordination compounds Amminebrom...
Text Solution
|
- Write the formulae for the follow Co-ordination compounds Dichlorido...
Text Solution
|
- Write the IUPAC names of the follow Co-ordination compounds. [Co(NH(...
Text Solution
|
- Write the IUPAC names of the follow Co-ordination compounds. [Co(NH(...
Text Solution
|
- Write the IUPAC names of the follow Co-ordination compounds. K(3)[Fe...
Text Solution
|
- Write the IUPAC names of the follow Co-ordination compounds. K(3)[Fe...
Text Solution
|
- Write the IUPAC names of the follow Co-ordination compounds. K(2)[Pd...
Text Solution
|