Text Solution
Verified by Experts
Topper's Solved these Questions
D AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|23 VideosCHEMISTRY IN EVERYDAY LIFE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Intex questions|5 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE|18 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-D AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS -INTEXT QUESTIONS
- Write the formulae for the follow Co-ordination compounds Amminebrom...
Text Solution
|
- Write the formulae for the follow Co-ordination compounds Dichlorido...
Text Solution
|
- Write the IUPAC names of the follow Co-ordination compounds. [Co(NH(...
Text Solution
|
- Write the IUPAC names of the follow Co-ordination compounds. [Co(NH(...
Text Solution
|
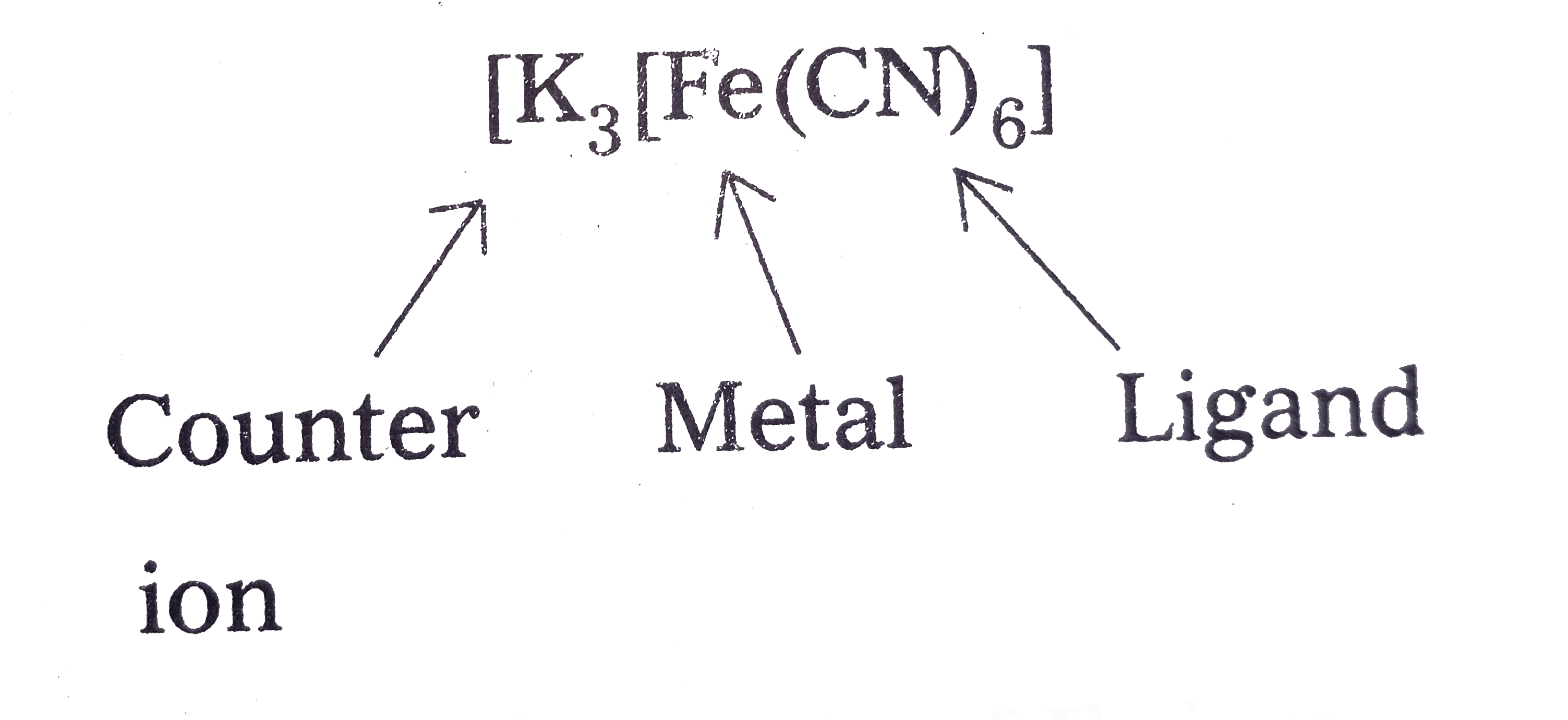
- Write the IUPAC names of the follow Co-ordination compounds. K(3)[Fe...
Text Solution
|
- Write the IUPAC names of the follow Co-ordination compounds. K(3)[Fe...
Text Solution
|
- Write the IUPAC names of the follow Co-ordination compounds. K(2)[Pd...
Text Solution
|
- Write the IUPAC names of the follow Co-ordination compounds. [Pt(NH(...
Text Solution
|
- Indicate the types of isomerism exhibited by the follow complexes and ...
Text Solution
|
- Indicate the types of isomerism exhibited by the follow complexes and ...
Text Solution
|
- Indicate the types of isomerism exhibited by the follow complexes and ...
Text Solution
|
- Indicate the types of isomerism exhibited by the follow complexes and ...
Text Solution
|
- Give evidence that [Co(NH(3))(5)Cl]SO(4) and [Co(NH(3))(5)SO(4)]Cl are...
Text Solution
|
- Explain on the basis of valence bond theory that [Ni(CN)(4)]^(2-) ion ...
Text Solution
|
- [NiCl(4)]^(2-) is paramagnetic while [Ni(CO)(4)] is diamgnetic though ...
Text Solution
|
- [Fe(H(2)O)(6)]^(3+) is strongly paramagnetic whereas [Fe(CN)(6)]^(3-) ...
Text Solution
|
- Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^(...
Text Solution
|
- Predict the number of unpaired electrons in the square planar [Pt(CN)(...
Text Solution
|
- The hexaquo manganese (II) ion contains five unpaired electrons, while...
Text Solution
|
- Calculate the overall complex dissociation equilibrium constant for th...
Text Solution
|