Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|29 VideosHALOALKANES AND HALOARENES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|22 VideosHALOALKANES AND HALOARENES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|13 VideosGENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE SAQ|1 VideosORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|54 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-HALOALKANES AND HALOARENES-SHORT ANSWER QUESTIONS
- Give the IUPAC names of the compound CH(3)CH(Cl) CH(I) CH(3)
Text Solution
|
- Give the IUPAC names of the compound ClCH(2) CH = CH CH(2) Br
Text Solution
|
- Give the IUPAC names of the compound (C Cl(3))(3) C Cl
Text Solution
|
- Give the IUPAC names of the compound CH(3) C (p-Cl-C(6) H(4))(2) CH...
Text Solution
|
- Write the structures of the following organic halides . 1-Bromo-4 se...
Text Solution
|
- Write the structures of the following organic halides . 2-Chloro-1-p...
Text Solution
|
- Write the structures of the following organic halides . p-bromochlor...
Text Solution
|
- Write the structures of the following organic halides . 4-t-butyl-3-...
Text Solution
|
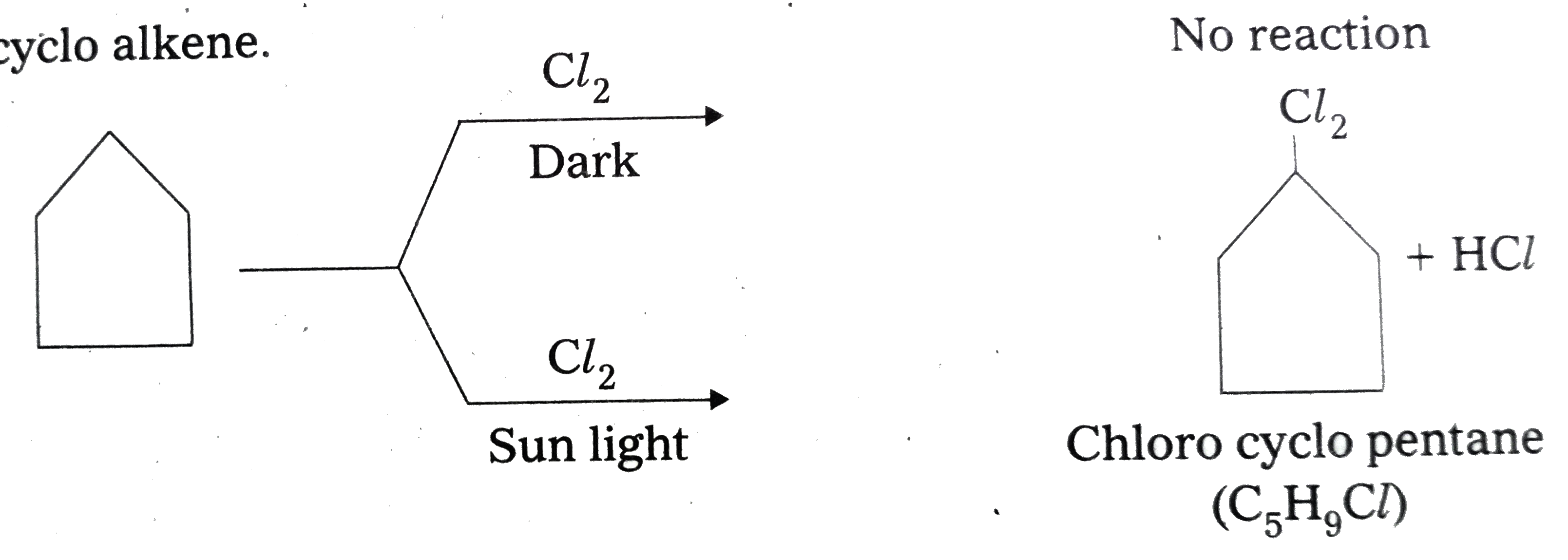
- A hydrocarbon C(5)H(10) does not react with chlorine in dark but gives...
Text Solution
|
- Which compound in following pairs will react faster in S(N) ^(2) rea...
Text Solution
|
- Which compound in following pairs will react faster in S(N) ^(2) rea...
Text Solution
|
- Predict the alkenes that would be formed in the reaction and identify ...
Text Solution
|
- Predict the alkenes that would be formed in the reaction and identify ...
Text Solution
|
- How will you carry out the conversion Ethane to bromomethene
Text Solution
|
- How will you carry out the conversion Toluene to benzyl alcohol
Text Solution
|
- Explain why the dipole moment of chlorobenzene is lower than that of c...
Text Solution
|
- Write the mechanism of the following reaction: nBuBr +KCNoverset(EtO...
Text Solution
|