Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VSAQ|1 VideosGENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE SAQ|1 VideosORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|54 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-HALOALKANES AND HALOARENES-SAQ
- Write the preparations of Alkyl halides .
Text Solution
|
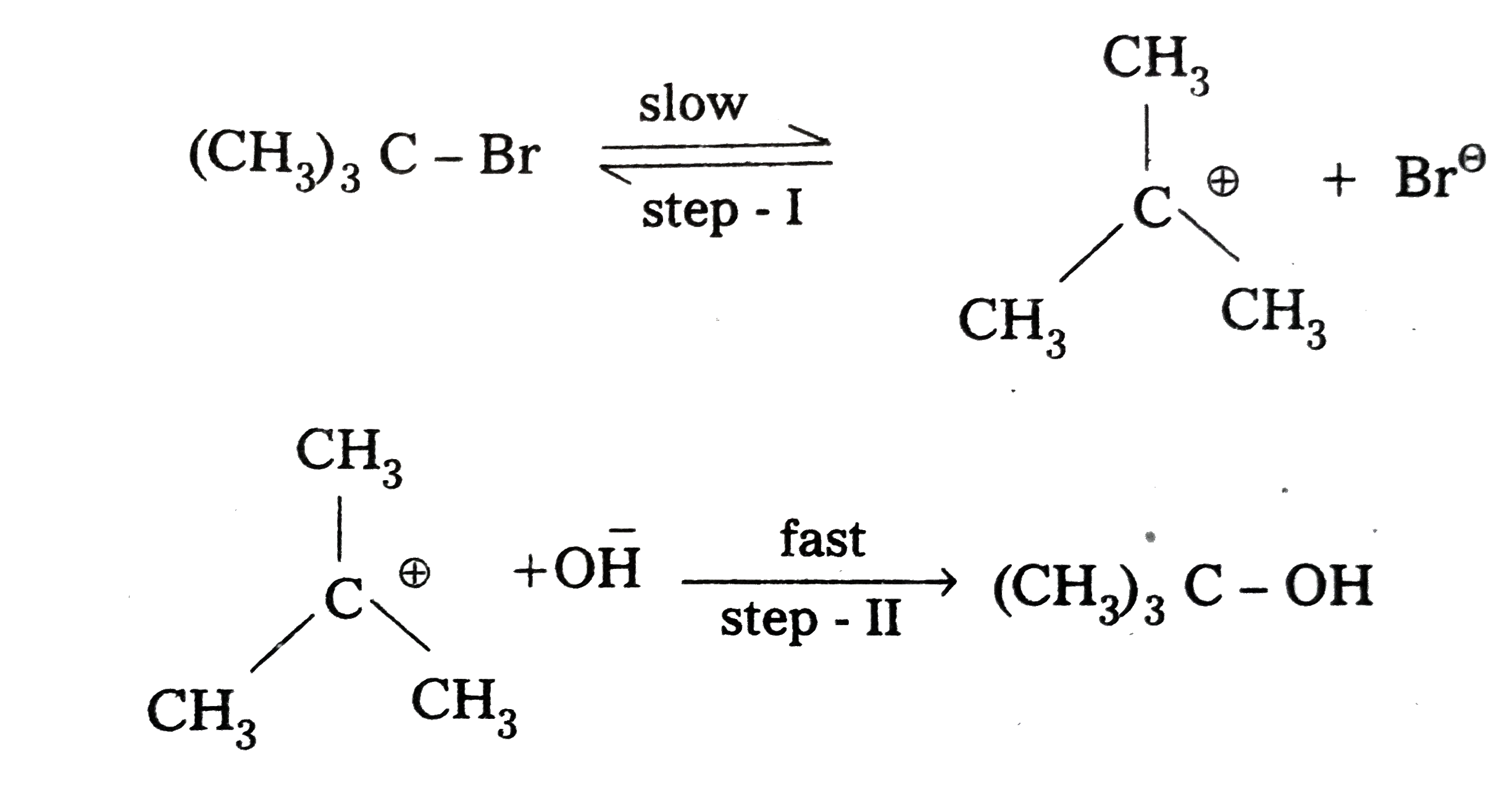
- Explain SN^(1) reaction
Text Solution
|
- Explain SN^(2) reaction
Text Solution
|
- What is Wurtz reaction ? Give equation .
Text Solution
|
- Explain different chemical properties of Alkyl halide .
Text Solution
|
- Explain Wurtz - Fitting reaction
Text Solution
|
- Explain Fittig reaction .
Text Solution
|
- Write any one method for the preparation of chloro benzene .
Text Solution
|
- Explain electrophilic substitution reactions of chloro benzene .
Text Solution
|