Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|88 VideosORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|54 VideosORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|36 VideosHALOALKANES AND HALOARENES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SAQ|9 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ORGANIC COMPOUNDS CONTAINING C,H AND O-SHORT ANSWER QUESTIONS
- Describe the Cross aldol condensation .
Text Solution
|
- Describe the Decarboxylation.
Text Solution
|
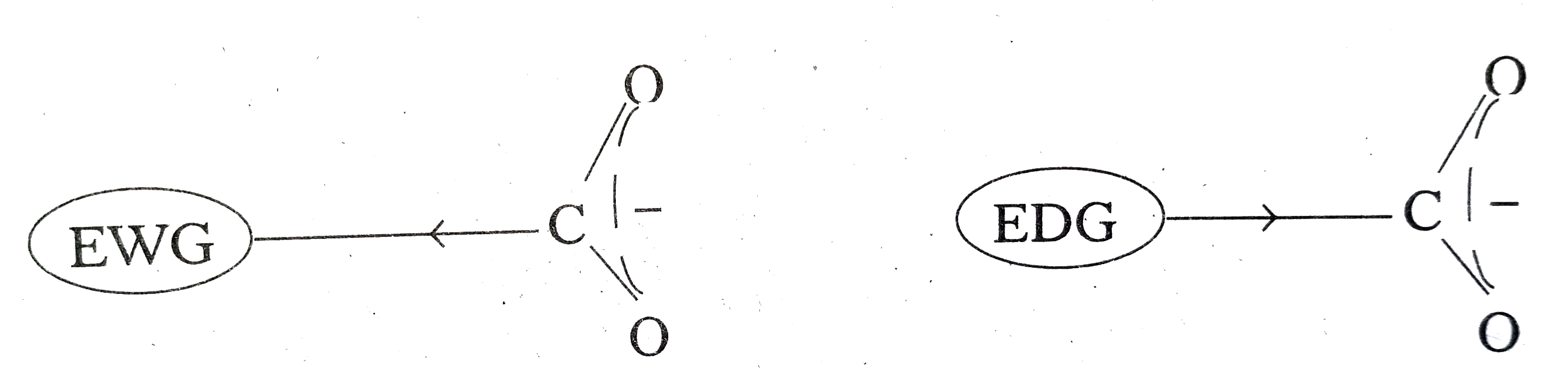
- Explain the role of electron withdrawing and electron releasing groups...
Text Solution
|
- Draw the structure of the Acetaldehydedimethylacetal .
Text Solution
|
- Draw the structure of the ethylene ketal of hexan-3-one.
Text Solution
|
- Draw the structure of the methyl hemiacetal of formaldehyde.
Text Solution
|
- An organic compound contains 69.77% carbon, 11.63% hydrogen and rest o...
Text Solution
|
- Explain Nucleophilic addition reaction mechanism of aldehydes and keto...
Text Solution
|
- Write any two methods for the preparation of Aldehydes.
Text Solution
|
- Write any two methods for the preparation of Ketones.
Text Solution
|
- Explain Clemenson's reduction and Wolf Kishmer reduction reactions.
Text Solution
|
- What is haloform reaction ? Give equation .
Text Solution
|
- What is Cannizaro reaction ? Give equation .
Text Solution
|
- What is HVZ reaction ? Give equation.
Text Solution
|
- Write any three methods for the preparation of Carboxylic acid.
Text Solution
|
- Explain Ring substitution reactions of aromatic carboxylic acids.
Text Solution
|
- How do you prepare the Acetyl chloride compound from acetic acid ?
Text Solution
|
- How do you prepare the Acetamide compound from acetic acid ?
Text Solution
|
- How do you prepare the Acetic anhydride compound from acetic acid ?
Text Solution
|
- How do you prepare the Ethyl alcohol compound from acetic acid ?
Text Solution
|