Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|54 VideosORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS|57 VideosHALOALKANES AND HALOARENES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SAQ|9 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ORGANIC COMPOUNDS CONTAINING C,H AND O-LONG ANSWER QUESTIONS
- How will you synthesise Cyclohexylmethanol using an alkyl halide by an...
Text Solution
|
- Show how will you synthesise: (i) 1-phenylethanol from a suitable al...
Text Solution
|
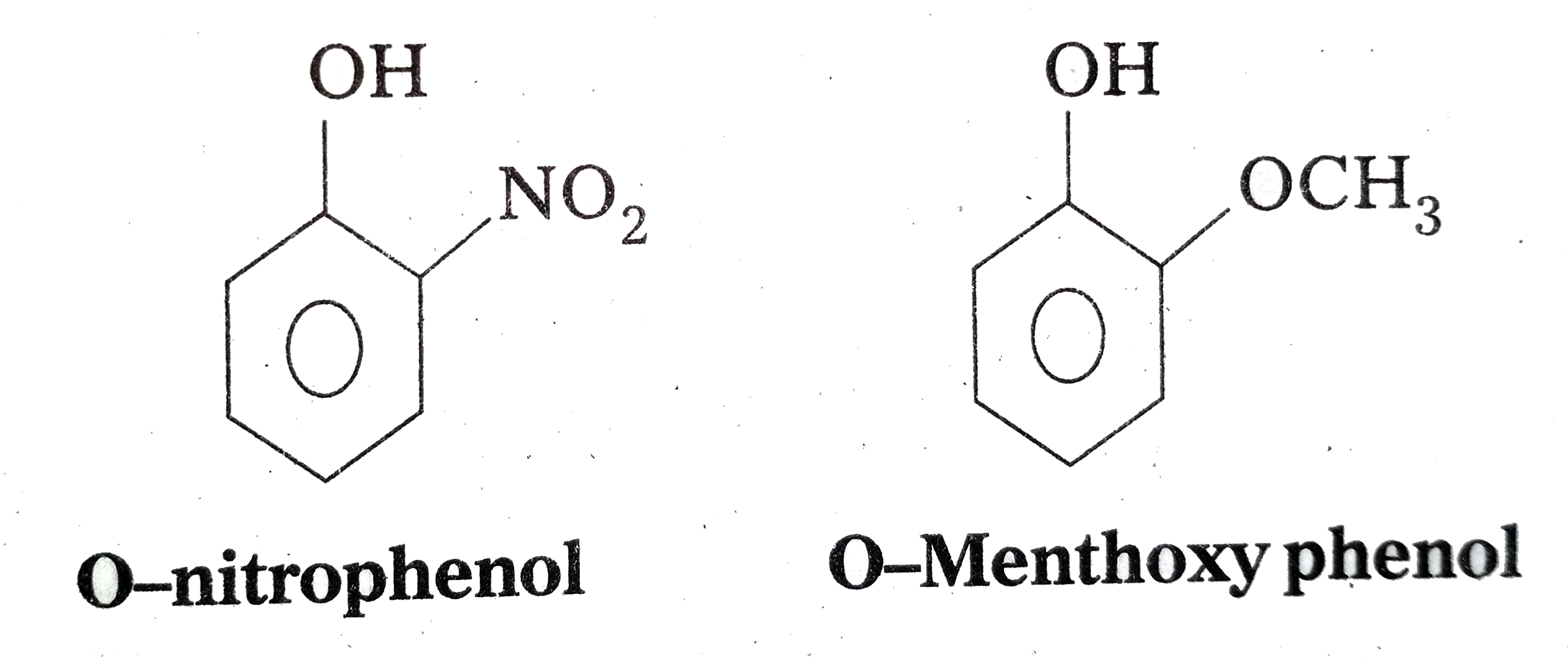
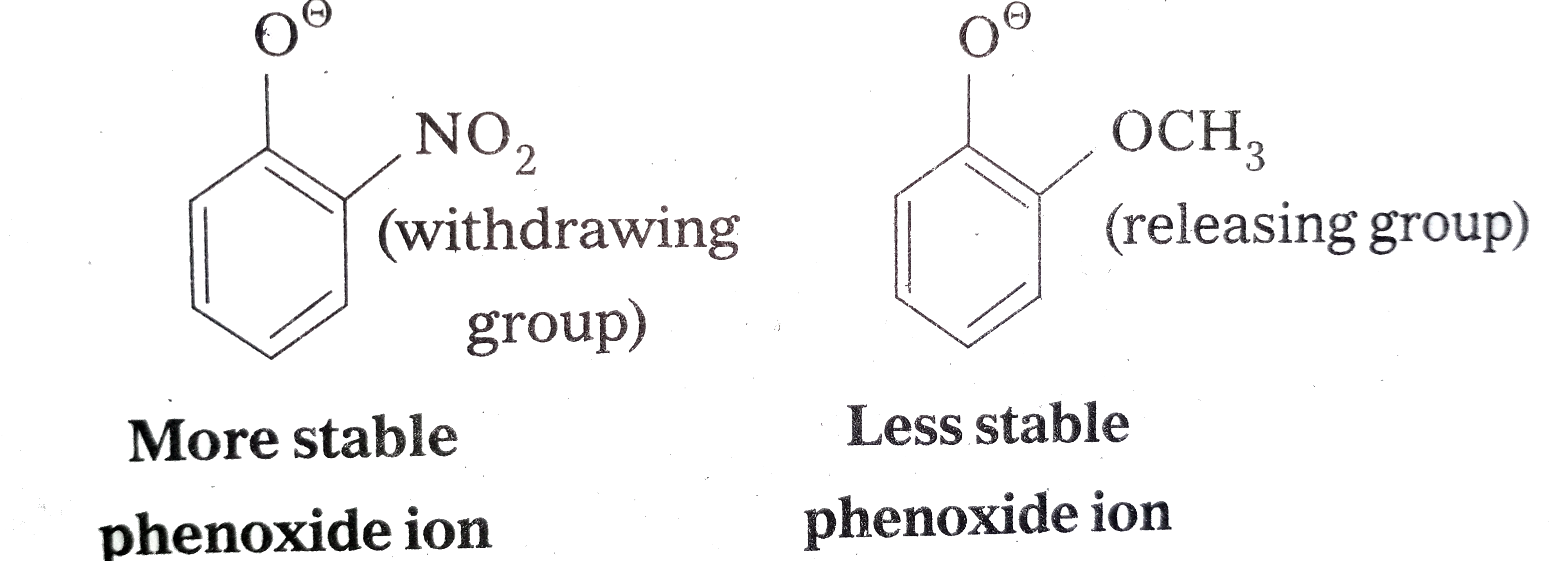
- Explain Ortho nitrophenol is more acidic than Ortho methoxyphenol.
Text Solution
|
- Explain OH group attached to benzene ring activates it towards electro...
Text Solution
|
- With a suitable example write equations for the Kolbe's reaction.
Text Solution
|
- With a suitable example write equations for the Reimer-Tiemann reacti...
Text Solution
|
- With a suitable example write equations for the Williamsons ether synt...
Text Solution
|
- How is Benzyl chloride to Benzyl alcohol conversions carried out ?
Text Solution
|
- How is Ethyl magnesium bromide to Propan-1-ol conversions carried out ...
Text Solution
|
- How is 2-Butanone to 2-Butanol conversions carried out ?
Text Solution
|
- Write the names of the reagents and equations for the preparation of t...
Text Solution
|
- Write the names of the reagents and equations for the preparation of t...
Text Solution
|
- Write the names of the reagents and equations for the preparation of t...
Text Solution
|
- Write the names of the reagents and equations for the preparation of t...
Text Solution
|
- How is 1-propoxyropane synthesized from propan-1-ol ? Write mechanism ...
Text Solution
|
- Explain the fact that in aryl alkyl ethers the alkoxy group activates ...
Text Solution
|
- Write equation of the Alkylation of anisole reaction.
Text Solution
|
- Write equations of the Nitration of anisole reactions.
Text Solution
|
- Write equation of the Friedel-Crafts acetylation of anisole reaction.
Text Solution
|
- Show how you would synthesize the alcohols from appropriate alkenes ?
Text Solution
|