Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|54 VideosORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS|57 VideosHALOALKANES AND HALOARENES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SAQ|9 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ORGANIC COMPOUNDS CONTAINING C,H AND O-LONG ANSWER QUESTIONS
- Show how you would synthesize the alcohols from appropriate alkenes ?
Text Solution
|
- Show how you would synthesize the alcohols from appropriate alkenes ?
Text Solution
|
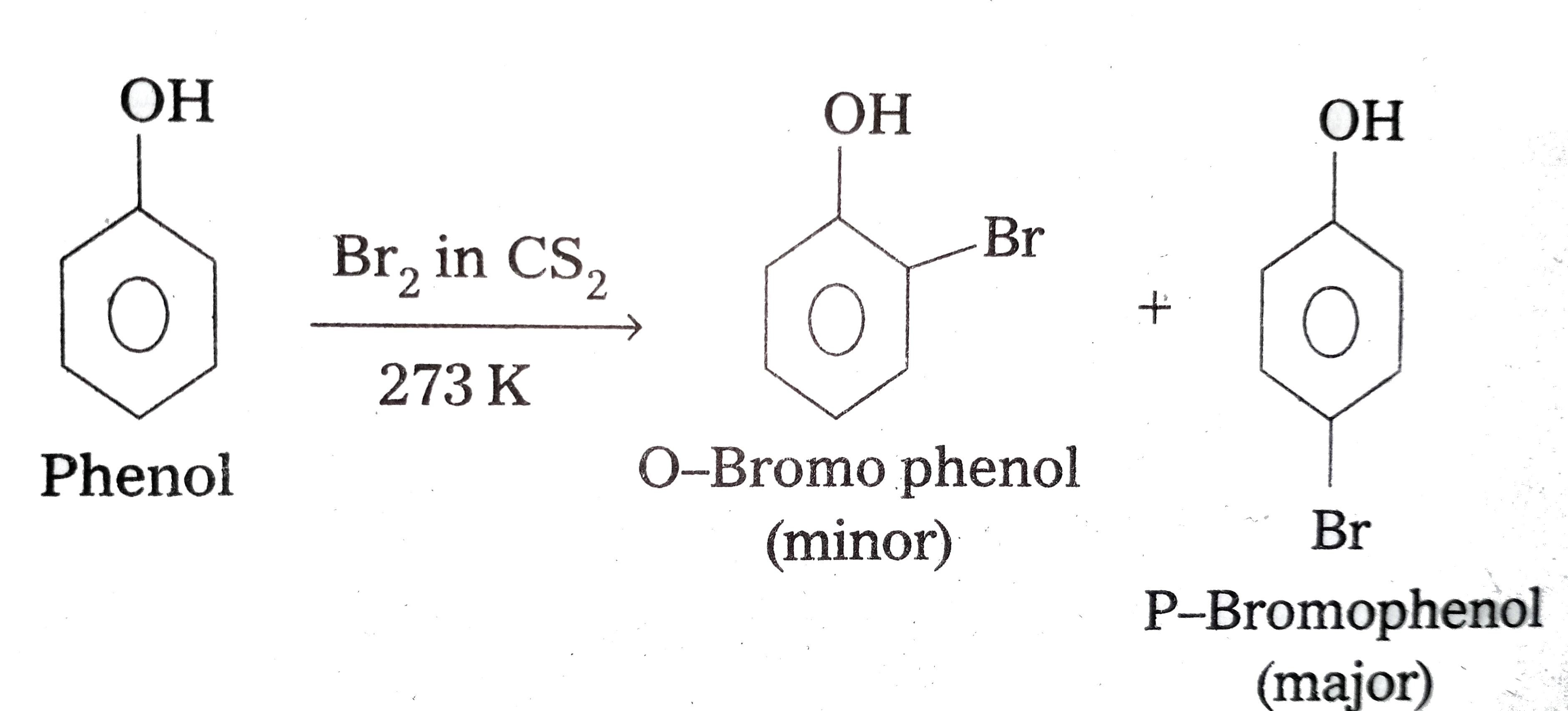
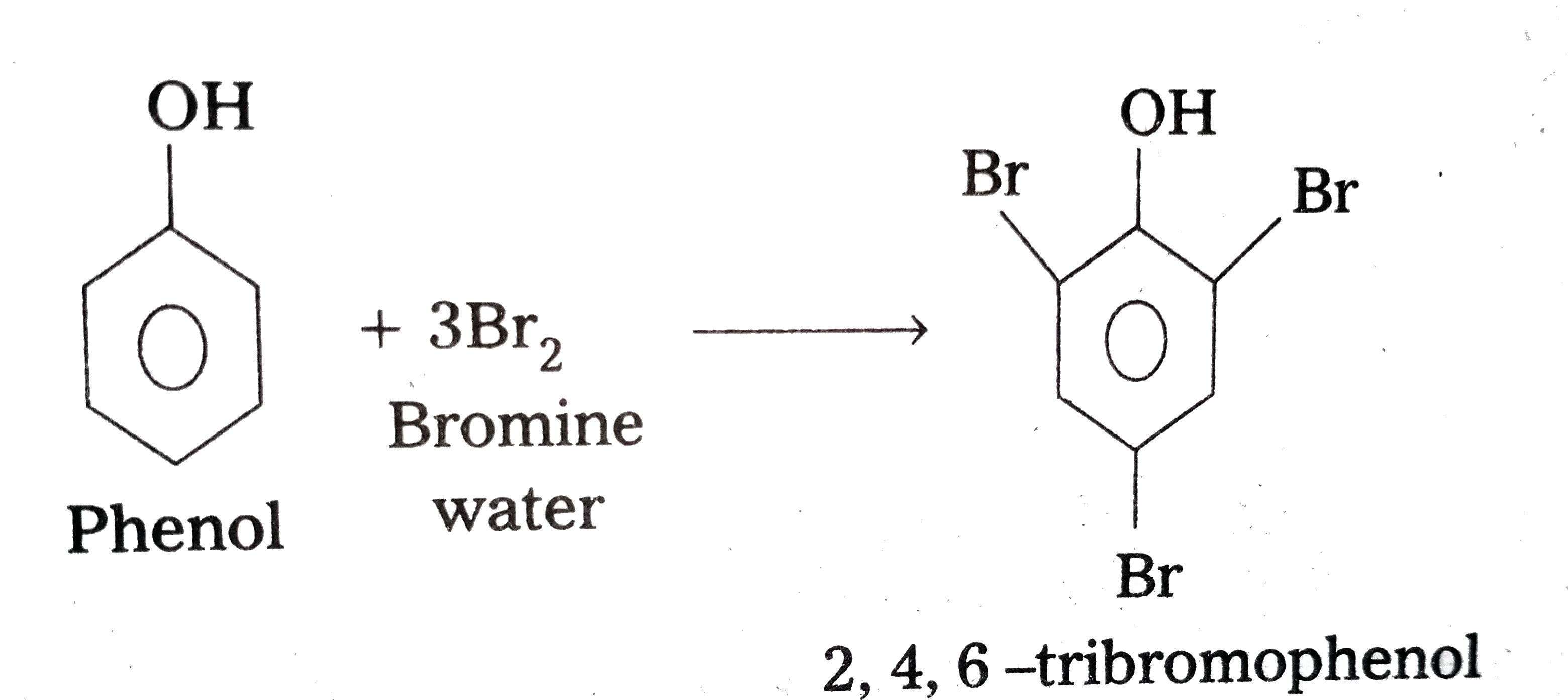
- Explain why phenol with bromine water forms 2,4,6-tribromophenol while...
Text Solution
|
- Explain the Cyanohydrin term. Give an example of the reaction .
Text Solution
|
- Explain the Acetal term. Give an example of the reaction .
Text Solution
|
- Explain the Semicarbazone term. Give an example of the reaction .
Text Solution
|
- Explain the Aldol term. Give an example of the reaction .
Text Solution
|
- Explain the Hemiacetal term. Give an example of the reaction .
Text Solution
|
- Explain the Oxime term. Give an example of the reaction .
Text Solution
|
- Write structures of the CH(3)CH(CH(3))CH(2)CH(2)CHO compound according...
Text Solution
|
- Write structures of the CH(3)CH(2)COCH(C(2)H(5))CH(2)CH(2)Cl compound ...
Text Solution
|
- Write structures of the CH(3)CH=CHCHO compound according to IUPAC syst...
Text Solution
|
- Write structures of the CH(3)COCH(2)COCH(3) compound according to IUPA...
Text Solution
|
- Draw the structure of the 3-Methylbutanal compound.
Text Solution
|
- Draw the structure of p-Nitropropiophenone compound.
Text Solution
|
- Draw the structure of p-Metylbenzaldehyde compound.
Text Solution
|
- Draw the structure of 3-Bromo-4-phenylpentanoic acid compound.
Text Solution
|
- Write the IUPAC names of the CH(3)CO(CH(2))(4)CH(3) ketones and Aldeh...
Text Solution
|
- Write the IUPAC names of the CH(3)CH(2)CHBrCH(2)CH(CH(3))CHO ketones ...
Text Solution
|
- Write the IUPAC names of the CH(3)(CH(2))(5)CHO ketones and Aldehydes...
Text Solution
|