Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|88 VideosHALOALKANES AND HALOARENES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SAQ|9 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ORGANIC COMPOUNDS CONTAINING C,H AND O-INTEXT QUESTIONS
- Classify the as primary , secondary and tertiary alcohols.
Text Solution
|
- Classify the following as primary, secondary and tertiary alcohols: ...
Text Solution
|
- Classify the as primary , secondary and tertiary alcohols.
Text Solution
|
- Identify the allylic alcohols in the above examples.
Text Solution
|
- Name the CH(3)-CH(2)-underset(CH(2)Cl)underset(|)CH-overset(CH(2)OH)ov...
Text Solution
|
- Name the CH(3)-underset(CH(3))underset(|)CH-CH(2)-underset(OH)underset...
Text Solution
|
- Name the compound according to IUPAC system.
Text Solution
|
- Name the H(2)C=CH-underset(OH)underset(|)CH-CH(2)-CH(2)-CH(3) compoun...
Text Solution
|
- Name the CH(3)-underset(CH(3))underset(|)C=underset(Br)underset(|)C-CH...
Text Solution
|
- Show how are the CH(3)-underset(CH(3))underset(|)CH-CH(2)OH alcohol p...
Text Solution
|
- Show how are the alcohol prepared by the reaction of a suitable Grig...
Text Solution
|
- Write structure of the products of the CH(3)-CH=CH(2) overset(H(2)O//H...
Text Solution
|
- Write structure of the products of the
Text Solution
|
- Write structure of the products of the CH(3)-CH(2)-underset(CH(3))und...
Text Solution
|
- Predict the major product of acid catalysed dehydration of 1-methycycl...
Text Solution
|
- Predict the major product of acid catalysed dehydration of butan-1-ol.
Text Solution
|
- Write the reactions of Williamson synthesis of 2-ethoxy-3-methypentane...
Text Solution
|
- Predict the products of the CH(3)-CH(2)-CH(2)-O-CH(3)-HBr to
Text Solution
|
- Predict the products of the
Text Solution
|
- Predict the products of the
Text Solution
|
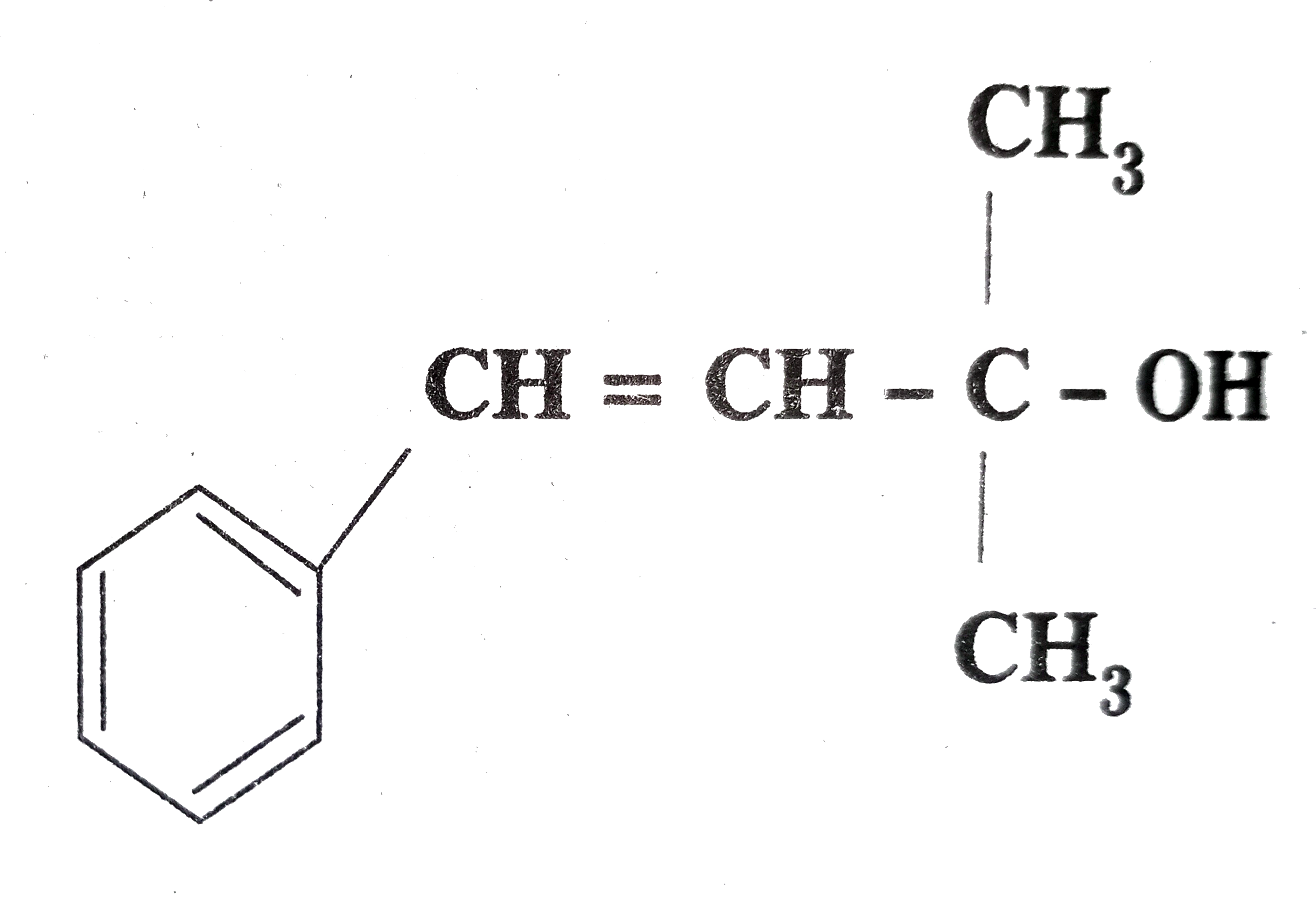 as primary , secondary and tertiary alcohols.
as primary , secondary and tertiary alcohols.