Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise short answer question|9 VideosATOMS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise long answer questions|3 VideosATOMS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise ADDITIONAL EXERCISES|24 VideosANDHRA PRADESH MARCH-2019
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SECTION-C|3 VideosCOMMUNICATION SYSTEM
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise TEXTUAL EXERCISES|8 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ATOMS-VERT SHORT ANSWER QUESTIONS
- What is the angular momentum of electron in the second orbit of Bohr's...
Text Solution
|
- Calculate the value of 'fine structure constant'
Text Solution
|
- What is the physical meaning of 'negative energy of an electron' ?
Text Solution
|
- Sharp lines are present in the spectrum of a gas. What does this indic...
Text Solution
|
- Name a physical quantity whose dimensions are the same as those of ang...
Text Solution
|
- What is the difference between alpha - particle and helium atom ?
Text Solution
|
- How is impact parameter related to the scattering angle?
Text Solution
|
- Among alpha, beta and gamma radiations, which get affected by the elec...
Text Solution
|
- What do you understand by the 'phrase ground state atom' ?
Text Solution
|
- Why does the mass of the nucleus not have any significance in scatteri...
Text Solution
|
- The Lyman series of hydrogen spectrum lies in the ultraviolet region. ...
Text Solution
|
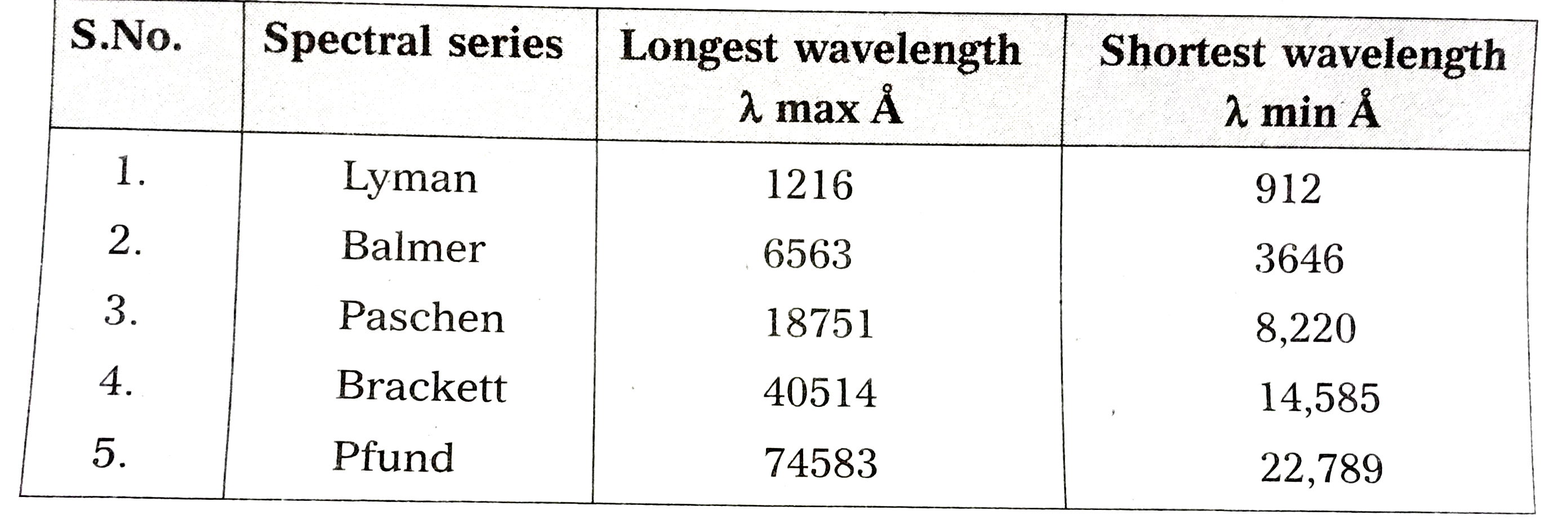
- Write down a table giving longest and shortest wavelengths of differen...
Text Solution
|
- The wavelengths of some of the spectral lines obtained in hydrogen spe...
Text Solution
|
- Give two drawbacks of Rutherford's atomic model.
Text Solution
|
- If the kinetic of revolving electron in an orbit is K, what is its pot...
Text Solution
|