Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Subject|17 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Single Correct|187 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Exercise 4.1|15 VideosALTERNATING CURRENT
CENGAGE PHYSICS|Exercise QUESTION BANK|65 VideosATOMS
CENGAGE PHYSICS|Exercise QUESTION BANK|40 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-ATOMIC PHYSICS-Exercise 4.2
- a. X-ray are electromagnetic waves because these are producted by the ...
Text Solution
|
- Find the cut off wavelength for the continuous X - rays coming from...
Text Solution
|
- Calculate the wavelength of K(alpha) line for the target made of tung...
Text Solution
|
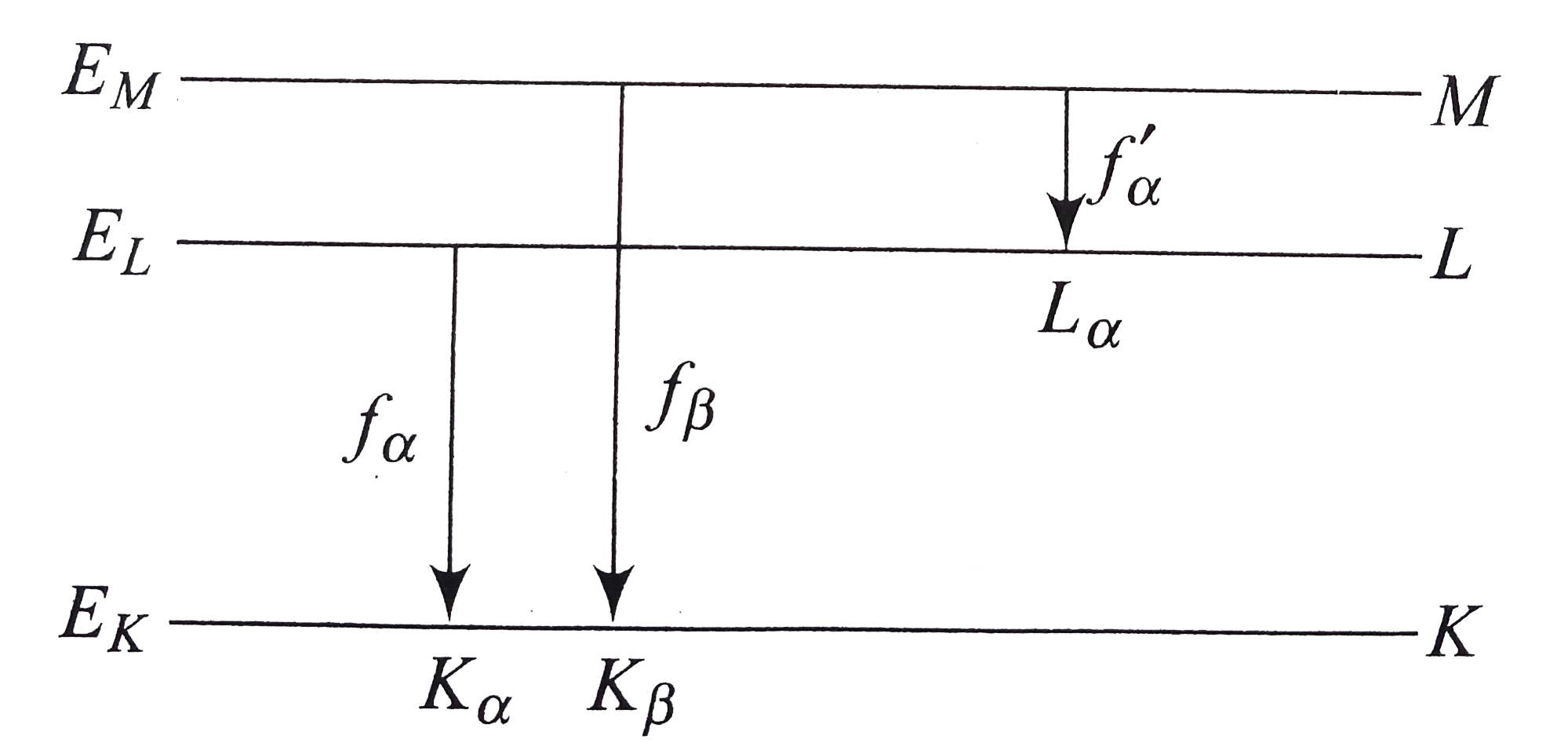
- Obtain a relation between the frequencies of K(oo) K(beta) and L(alpha...
Text Solution
|
- An X-ray tube operates at 20k V. Find the maximum speed of the electro...
Text Solution
|
- (a) An X-ray tube produces a continuous spectrum of radiation with its...
Text Solution
|
- The wavelength of the characteristic X-ray K(alpha) line emitted from...
Text Solution
|
- If the shorts series limit of the balmer series for hydrogen is 3644 Å...
Text Solution
|
- A material whose K -absorption edge is 0.2 Å is irradiated by X-ray of...
Text Solution
|
- Calculate the wavelength of the emitted characteristic X-ray from a tu...
Text Solution
|
- A potential diffrence , the 20kv is applied across an X- ray s tube ....
Text Solution
|
- The wavelength of k(alpha) X- rays produced by an X - rays tube is 0....
Text Solution
|