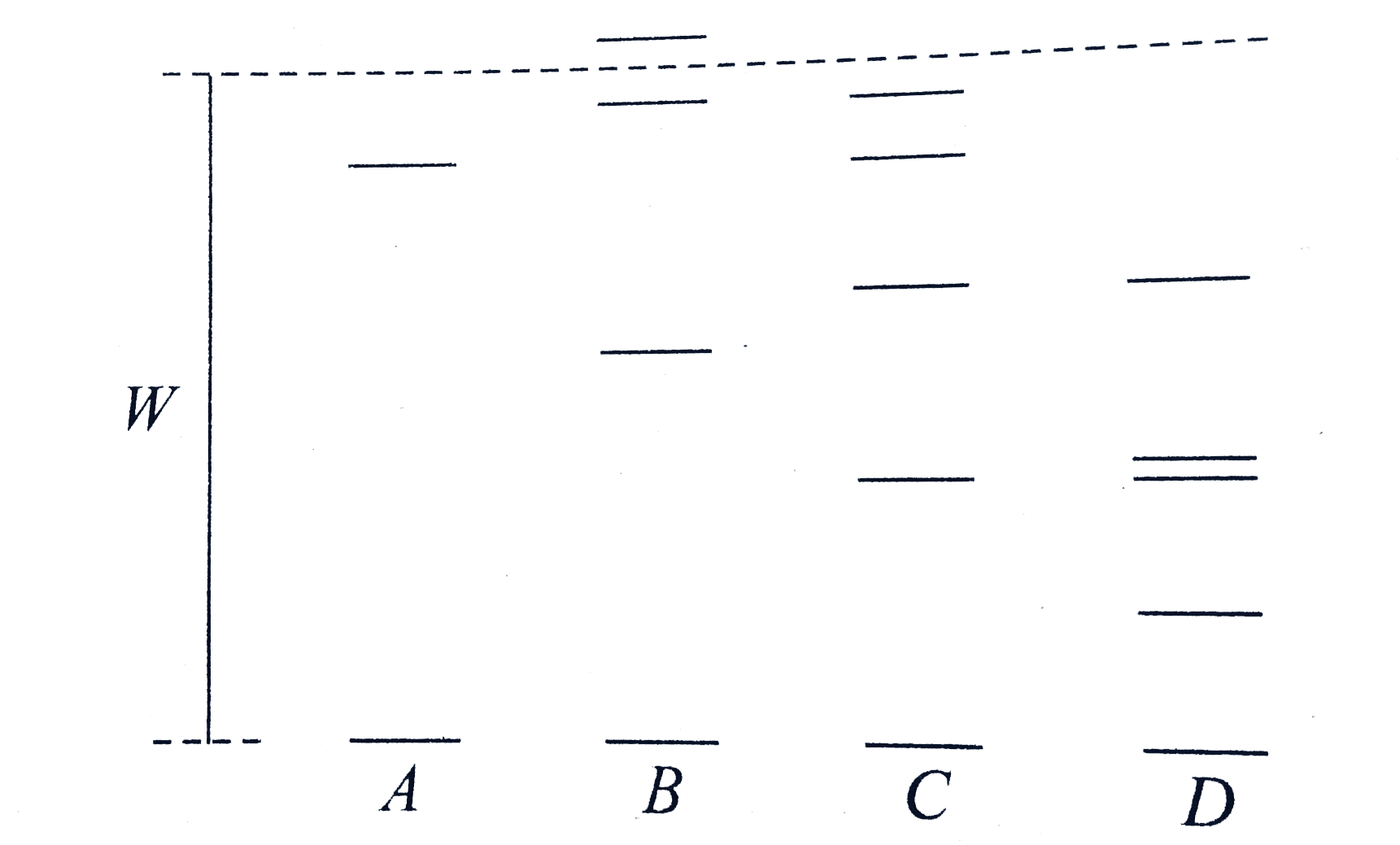
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Multiple Correct|13 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Linked Comprehension|62 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Subject|17 VideosALTERNATING CURRENT
CENGAGE PHYSICS|Exercise QUESTION BANK|65 VideosATOMS
CENGAGE PHYSICS|Exercise QUESTION BANK|40 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-ATOMIC PHYSICS-Single Correct
- Figure represent some of the lower energy level of the hydrogen atom i...
Text Solution
|
- The orbiting v(n) of e^(bar) in the nth orbit in the case of positroni...
Text Solution
|
- Figure shown the electron energy level , referred to the ground state ...
Text Solution
|
- When an electron jumps from a level n = 4 to n = 1, the momentum of t...
Text Solution
|
- Check the corretness of the following statement about the Bohr model o...
Text Solution
|
- when a hydrogen atom is raised from the ground state to an excited sta...
Text Solution
|
- As the electron in the Bohr orbit is hydrogen atom passes from state n...
Text Solution
|
- Consider a spectral line resulting n = 5 to n = 1, in the atom and los...
Text Solution
|
- Which of the following statement is true regarding Bohr's model of hyd...
Text Solution
|
- In the Bohr model of a hydrogen atom, the centripetal force is furnish...
Text Solution
|
- Hydrogen atoms are excited from ground state of the principle quantum ...
Text Solution
|
- When an electron jumps from n(1) th orbit to n(2) th orbit, the energ...
Text Solution
|
- In Bohr's model of hydrogen atom, let PE represents potential energy a...
Text Solution
|
- if the electron in hydrogen orbit jumps form third orbit to second or...
Text Solution
|
- The energy change is greatest for a hydrogen atom when its state chang...
Text Solution
|
- The electron in a hydrogen atom jumps from ground state to the higher ...
Text Solution
|
- Energy of an electron in an excited hydrogen atom is -3.4eV. Its angua...
Text Solution
|
- The wavelength of the first line of Lyman series in hydrogen atom is...
Text Solution
|
- Energy E of a hydrogen atom with principle quantum number n is given b...
Text Solution
|
- If 13.6 eV energy is required to ionized the hydrogen atom then the en...
Text Solution
|