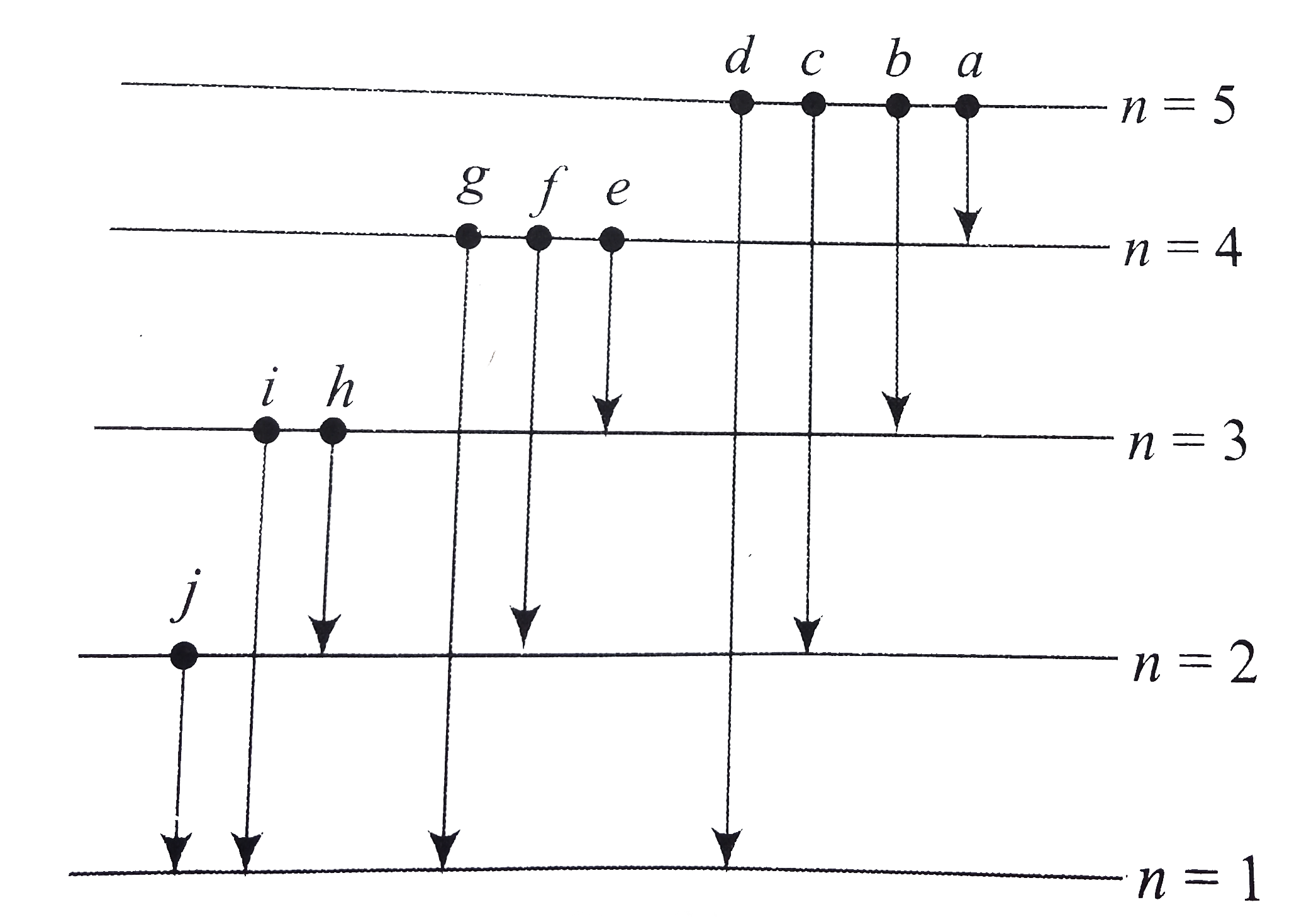
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Multiple Correct|13 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Linked Comprehension|62 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Subject|17 VideosALTERNATING CURRENT
CENGAGE PHYSICS|Exercise QUESTION BANK|65 VideosATOMS
CENGAGE PHYSICS|Exercise QUESTION BANK|40 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-ATOMIC PHYSICS-Single Correct
- The power of an X-ray tube is 16 W. If the potential difference applie...
Text Solution
|
- A hydrogen like atom (atomic number Z) is in a higher excited state o...
Text Solution
|
- Hydrogen aton in a sample are excited to n = 5 state and it is found t...
Text Solution
|
- A sample of hydrogen atom is in excited state (all the atoms ). The ph...
Text Solution
|
- Mark out the correct statement regarding X-rays.
Text Solution
|
- An electron collides with a hydrogen atom in its ground state and exci...
Text Solution
|
- Electron in a hydrogen-like atom (Z = 3) make transition from the fort...
Text Solution
|
- In which of the following transition will the wavelength be minimum ?
Text Solution
|
- An electron with kinetic energy 5eV is incident on a hydrogen atom in ...
Text Solution
|
- If the average life time of an excited state of hydrogen is of the ord...
Text Solution
|
- An electron of energy 11.2 eV undergoes an inelastic collision with a...
Text Solution
|
- The recoil speed of hydrogen atom after it emits a photon in going fro...
Text Solution
|
- A beam of 13.0 eV electrons is used to bombard gaseous hydrogen. The s...
Text Solution
|
- The wavelength of the spectral line that corresponds to a transition i...
Text Solution
|
- The angular momentum of an electron in hydrogen atom is 4 h// 2 pi. Ki...
Text Solution
|
- different between nth and (n + 1) th Bohr's radius of hydrogen atom is...
Text Solution
|
- k(a) wavelength emitted by an atom of atomic number Z= 11 is lambda f...
Text Solution
|
- A proton of mass m moving with a speed v(0) apporoches a stationary pr...
Text Solution
|
- If an X-ray tube operates at the voltage of 10kV, find the ratio of th...
Text Solution
|
- The potential different across the Coolidge tube is 20 kV and 10 m A c...
Text Solution
|