A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Multiple Correct|13 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Linked Comprehension|62 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Subject|17 VideosALTERNATING CURRENT
CENGAGE PHYSICS|Exercise QUESTION BANK|65 VideosATOMS
CENGAGE PHYSICS|Exercise QUESTION BANK|40 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-ATOMIC PHYSICS-Single Correct
- If the potential difference applied across a Coolidge tube is increase...
Text Solution
|
- An X-ray tube is operating at 150 kV and 10 mA. If only 1% of the ele...
Text Solution
|
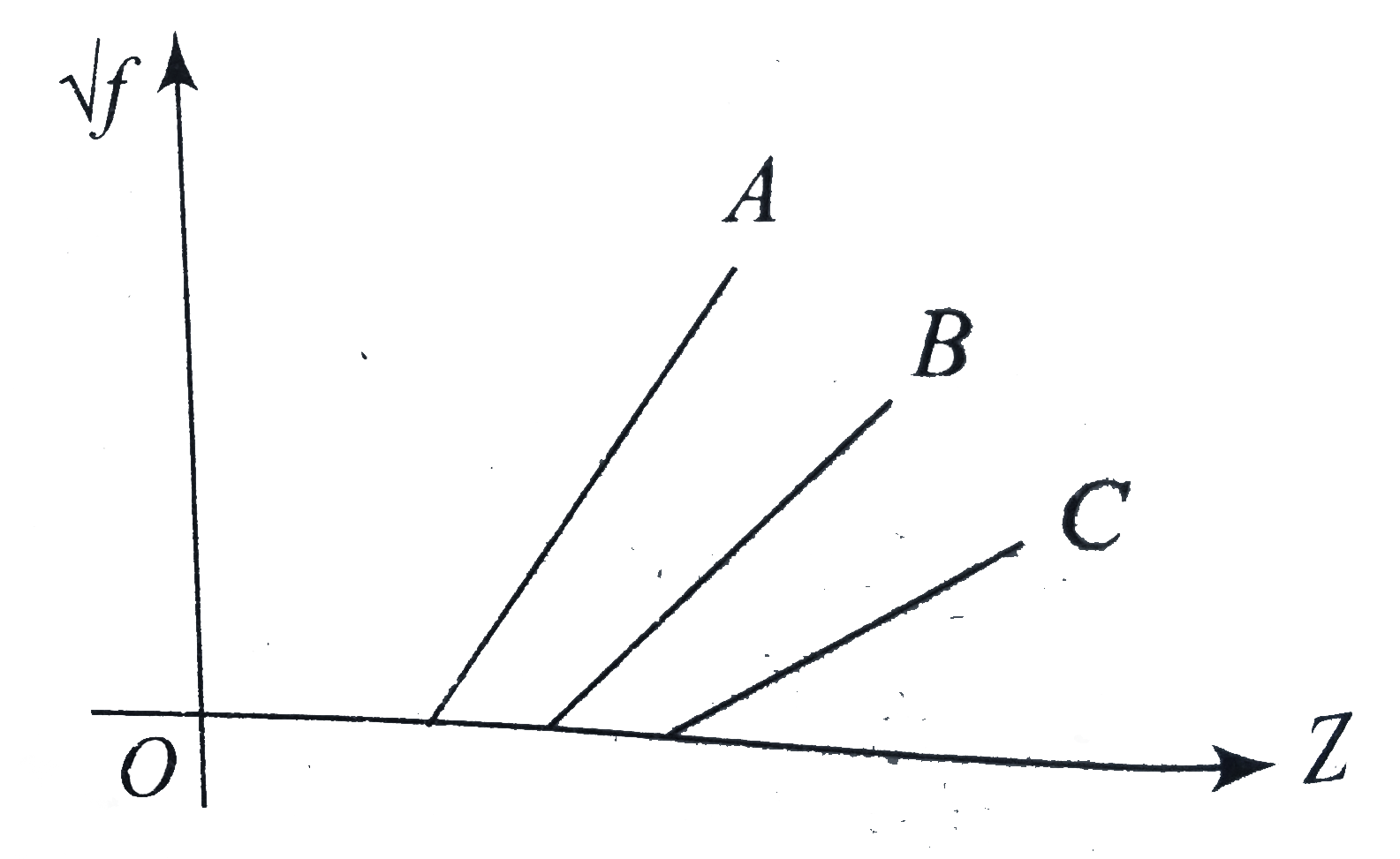
- Figure shown Moseley's plot between sqrt f and Z where f is the freque...
Text Solution
|
- An electron in the ground state of hydrogen has an angular momentum L(...
Text Solution
|
- The approximate value of quantum number n for the circular orbit o...
Text Solution
|
- If element with particle quantum number ngt4 were not allowed in natu...
Text Solution
|
- The velocity of an electron in the first orbit of H atom is v . The ve...
Text Solution
|
- The recoil speed of a hydrogen atom after it emits a photon is going...
Text Solution
|
- If a hydrogen atom emit a photon of energy 12.1 eV , its orbital angul...
Text Solution
|
- An electron of kinetic energy K collides elastically with a stationary...
Text Solution
|
- The wavelength of k(alpha) X- rays produced by an X - rays tube is 0....
Text Solution
|
- Let the potential energy of the hydrogen atom in the ground state be z...
Text Solution
|
- When photon of wavelength lambda(1) are incident on an isolated shere ...
Text Solution
|
- The ionization of the ionized sodium atom N a^(10) is:
Text Solution
|
- The shortest wavelength produced in an X-ray tube operating at 0.5 mil...
Text Solution
|
- If the radiation of Mo (Z = 42) has a wevelength of 0.71Å, calculate w...
Text Solution
|
- A beam of electron accelerated by a large potential difference V is ...
Text Solution
|
- The minimum kinetic energy required for ionization of a hydrogen atom ...
Text Solution
|
- Magnetic field at the center (at nucleus) of the hydrogen like atom ("...
Text Solution
|
- Magnetic moment due to the motion of the electron in nth energy of hyd...
Text Solution
|