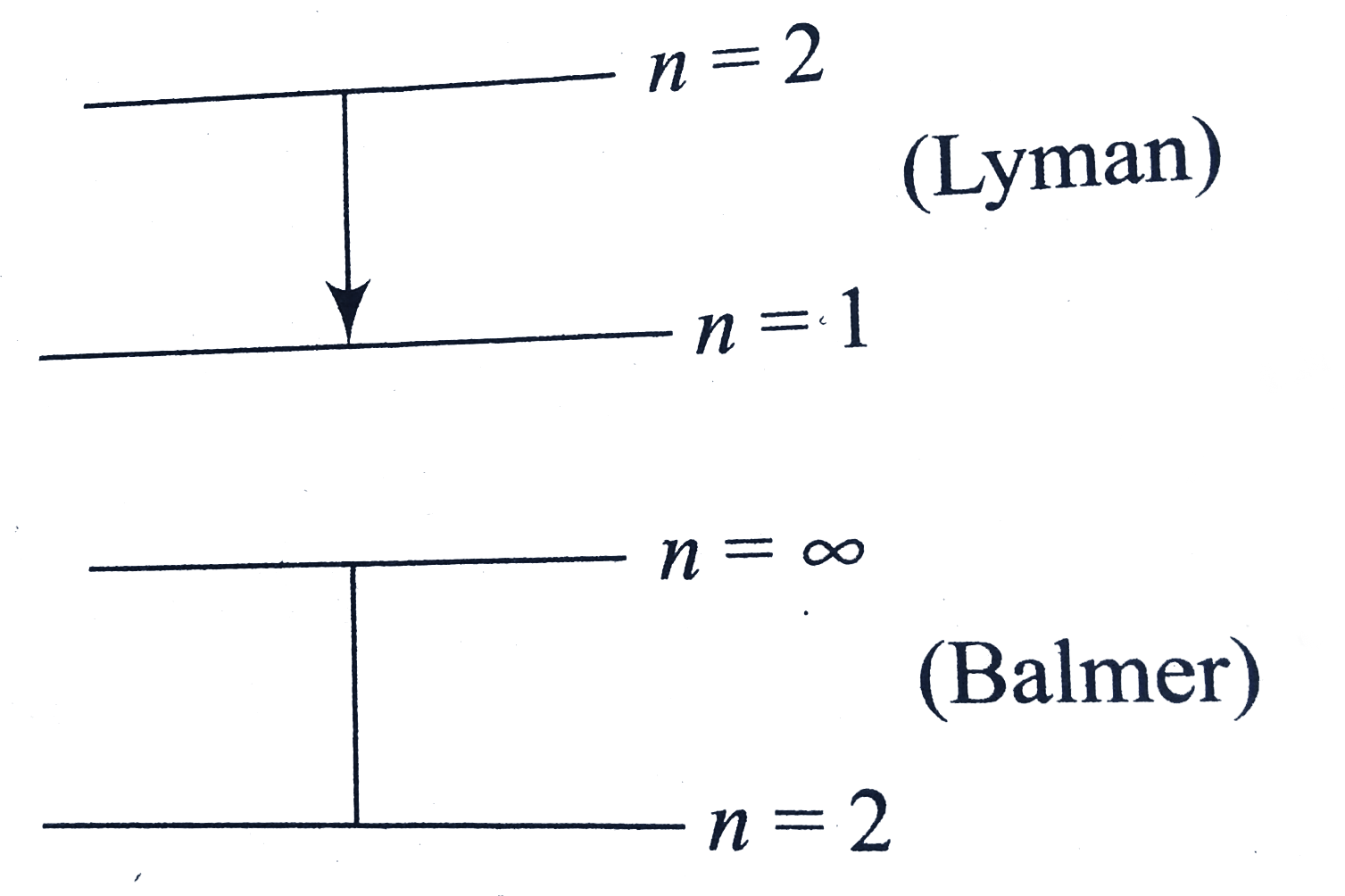
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Linked Comprehension|62 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Integer|4 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Single Correct|187 VideosALTERNATING CURRENT
CENGAGE PHYSICS|Exercise QUESTION BANK|65 VideosATOMS
CENGAGE PHYSICS|Exercise QUESTION BANK|40 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-ATOMIC PHYSICS-Multiple Correct
- Which of the following statements are true?
Text Solution
|
- An X-ray tube is operated at 6.6 kV. In the continuous spectrum of th...
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- According to Einstein's theory of relativity, mass can be converted in...
Text Solution
|
- X-ray from a tube with a target A of atomic number Z shows strong K l...
Text Solution
|
- In Bohr model of the hydrogen atom, let R,v and E represent the radiu...
Text Solution
|
- An X-ray tube is operated at 50 kV and 20 m A. The target material of ...
Text Solution
|
- For a cartain metal, the K obsorption edge is at 0.72 Å. The wavelengt...
Text Solution
|
- The third line of the Balmer series spectrum of a hydrogen-like ion of...
Text Solution
|
- Assertion: In a hydrogen atom energy of emitted photon corresponding t...
Text Solution
|
- Statement I: In an X-ray tube , if the energy with which an electron s...
Text Solution
|
- Statement I : The energy of a He^(+) ion for a given n is almost exact...
Text Solution
|
- Hydrogen is the simplest atom of nature. There is one proton in its nu...
Text Solution
|