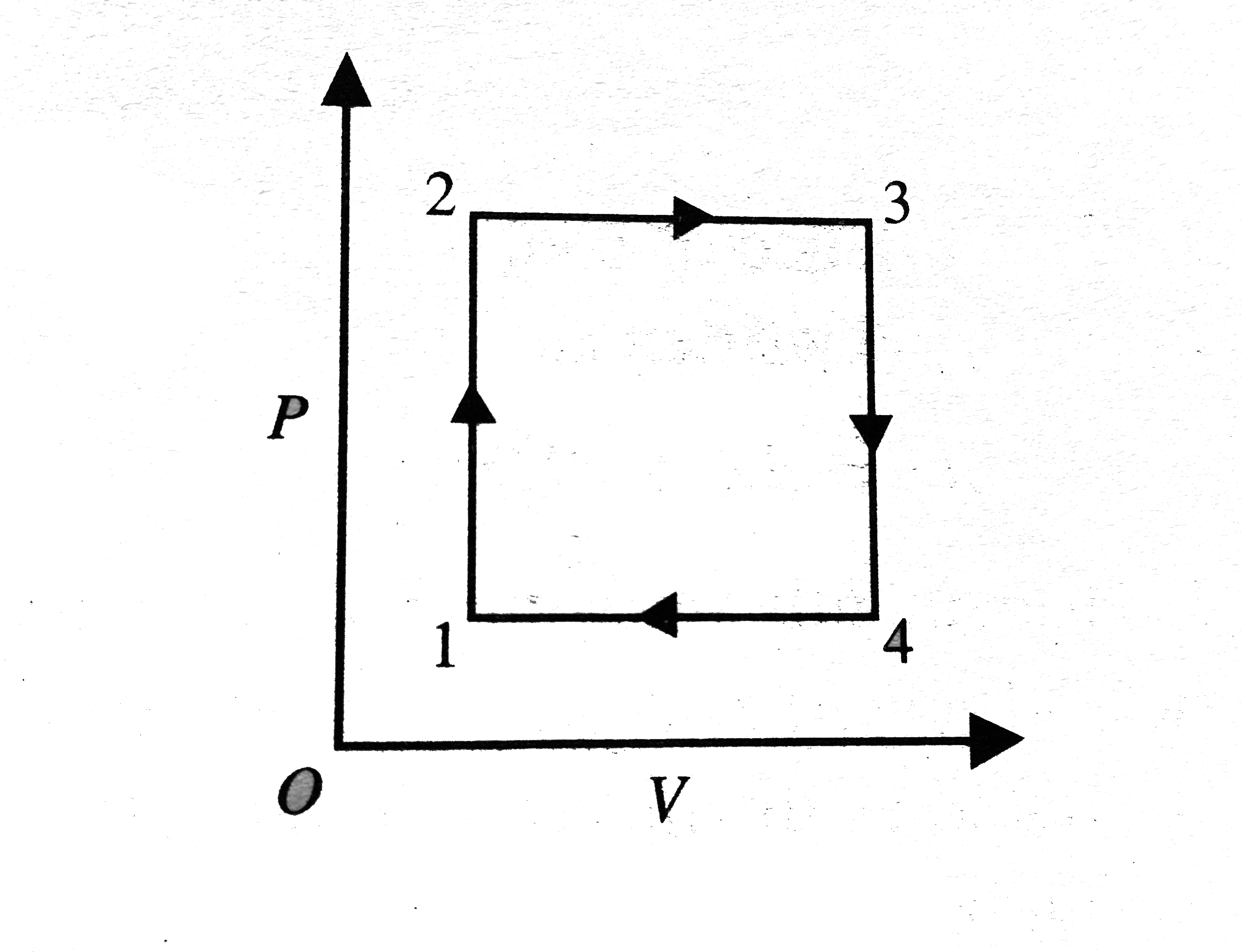Text Solution
Verified by Experts
Topper's Solved these Questions
KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Exercise 2.2|28 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Subjective|22 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Solved Examples|14 VideosKINETIC THEORY OF GASES
CENGAGE PHYSICS|Exercise Compression|2 VideosLINEAR AND ANGULAR SIMPLE HARMONIC MOTION
CENGAGE PHYSICS|Exercise Multiple Correct Answer Type|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS-Exercise 2.1
- The average kinetic energy per molecule of all monatomic gases is the ...
Text Solution
|
- What happens to the random motion when an ideal gas undergoes free exp...
Text Solution
|
- The highest vacuum attained so far is of the order of 10^(-11) mm of m...
Text Solution
|
- A thermally insulated vessel with gaseous nitrogen at a temperature of...
Text Solution
|
- Find the molar mass and the number of degrees of freedom of molecules ...
Text Solution
|
- At 127^(@)C and 1.00 xx 10^(-2) atm pressure, the density of a gas is ...
Text Solution
|
- The mass of a gas molecule can be computed form the specific heat at c...
Text Solution
|
- In a certain (2/5) th of the energy of molecules is associated with th...
Text Solution
|
- If the water molecules in 1 g of water were distributed uniformaly ove...
Text Solution
|
- How many degress of freedom have the gas molecules, if under standard ...
Text Solution
|
- The temperature of a gas consisting of rigid diatomic moleculoes is T ...
Text Solution
|
- The molar specific heat capacity of all monatomic gases is the same. I...
Text Solution
|
- One mole of a mono-atomic ideal gas is mixed with one mole of a diatom...
Text Solution
|
- A gas has been subjected to isochoric-isobaric processes 1-2-3-4-1 see...
Text Solution
|
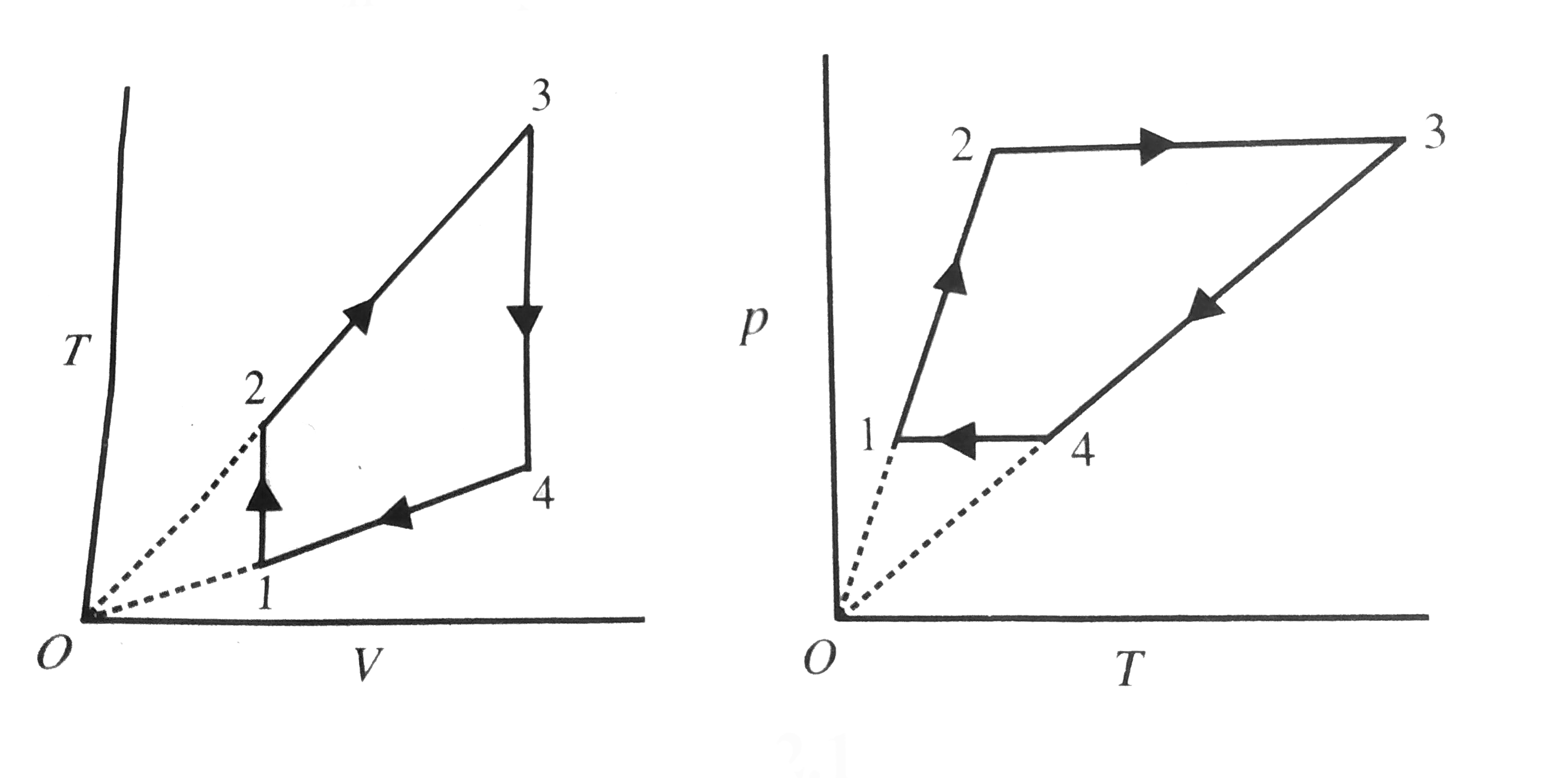
- A gas has been subjected to an isothermal-isochromic cycle 1-2-3-4-1 s...
Text Solution
|
- Calculate gamma of a gaseous mixture consisting of 3 moles of nitrogen...
Text Solution
|
- A closed container of volume 0.02m^3contains a mixture of neon and arg...
Text Solution
|
- Equal masses of air are sealed in two vessels, one of volume V(0) and ...
Text Solution
|
- A glass container encloses a gas a pressure of 8 xx 10^(5) pa and 300 ...
Text Solution
|
- Calculate the number of molecules in 1 cc of an ideal gas at 27^(@)C a...
Text Solution
|