Text Solution
Verified by Experts
Topper's Solved these Questions
KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Subjective|22 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Single Correct|140 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Exercise 2.1|20 VideosKINETIC THEORY OF GASES
CENGAGE PHYSICS|Exercise Compression|2 VideosLINEAR AND ANGULAR SIMPLE HARMONIC MOTION
CENGAGE PHYSICS|Exercise Multiple Correct Answer Type|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS-Exercise 2.2
- Explain why the temperature of a gas drops in an adiabatic expansion,...
Text Solution
|
- In what process is the heat added entirely converted into internal ene...
Text Solution
|
- When a gas is compressed adiabaticlly, it becomes more elasitc. Is thi...
Text Solution
|
- A cylinder contains 3 moles of oxygen at a temperature of 27^(@)C. The...
Text Solution
|
- What amount of heat is to be transferred to nitrogen in an isobaric he...
Text Solution
|
- As a result of the isobaric heating by DeltaT=72K, one mole of a certa...
Text Solution
|
- A certain mass of a gas is compressed first adiabatically, and then is...
Text Solution
|
- A cylindrical vessel of 28 cm diameter contains 20 g of nitrogen compr...
Text Solution
|
- One mole of oxygen is heated at constant pressure starting at 0^(@)C. ...
Text Solution
|
- An ideal gas expands from an initial temperature T(1) to a final tempe...
Text Solution
|
- An ideal gas whose adiabatic exponent equals gamma expands so that the...
Text Solution
|
- On mole of argon expands polytropically, the polytropic constant being...
Text Solution
|
- Find out the work done in the given graph. Also draw the corresponding...
Text Solution
|
- Two moles of a diatomic gas at 300 K are kept in a nonconducting conta...
Text Solution
|
- A sample of an ideal gas is taken through the cyclic process abca . It...
Text Solution
|
- The internal energy of a monatomic ideal gas is 1.5 nRT.One mole of he...
Text Solution
|
- A sample of ideal gas (gamma = 1.4) is heated at constant pressure. If...
Text Solution
|
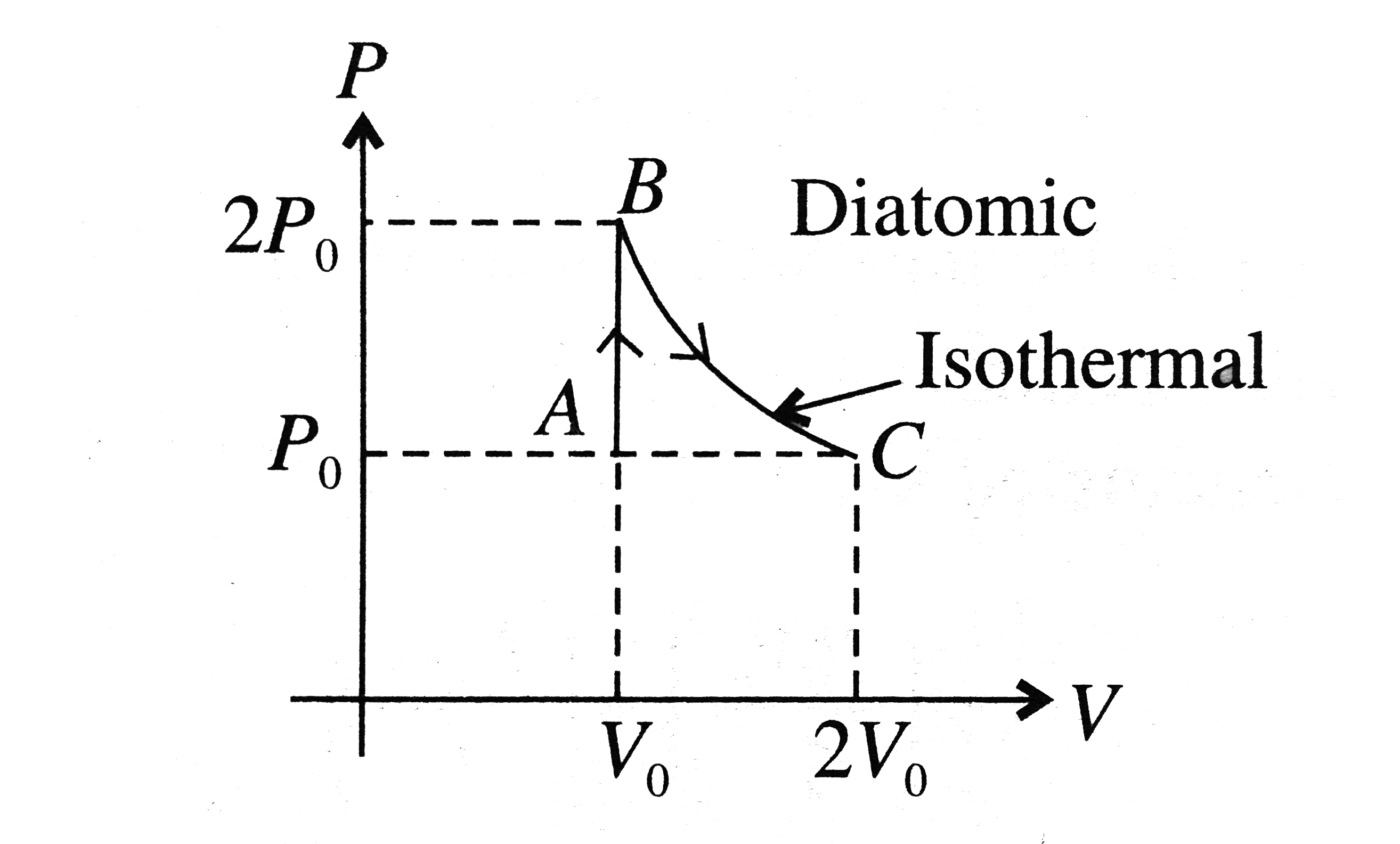
- P-V curve of a diatomic gas is shown in the Fig. Find the total heat g...
Text Solution
|
- A monoatomic gas is enclosed in a nonconducting cylinder having a pist...
Text Solution
|
- A nonconducting cylinder having volume 2 V(0) is partitioned by a fixe...
Text Solution
|