Text Solution
Verified by Experts
Topper's Solved these Questions
KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Single Correct|140 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Multiple Corrects|29 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Exercise 2.2|28 VideosKINETIC THEORY OF GASES
CENGAGE PHYSICS|Exercise Compression|2 VideosLINEAR AND ANGULAR SIMPLE HARMONIC MOTION
CENGAGE PHYSICS|Exercise Multiple Correct Answer Type|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS-Subjective
- Fig. shows the variation of internal energy (U) with the pressure (P) ...
Text Solution
|
- Two moles a monatomic gas in state A having critical pressure P(0) and...
Text Solution
|
- A gaseous mixture enclosed in a vessel consists of one gram mole of a ...
Text Solution
|
- A gas expands in a piston-cylinder device from volume V(1) to V(2), th...
Text Solution
|
- Find the minimum attainable pressure of an ideal gas in the process T ...
Text Solution
|
- Air is contained in a piston-cylinder arrangement as shown in Fig. wit...
Text Solution
|
- Consider the cyclic process ABCA, shown in figure, performed on a samp...
Text Solution
|
- Figure shows the variation in the internal energy U with the volume V ...
Text Solution
|
- One mole of a gas is carried through the cycle shown in Fig. The gas e...
Text Solution
|
- One mole of a monatomic gas is taken from a point A to another point B...
Text Solution
|
- Figure shows an adiabatic cylindrical tube of volume (V0) divided in t...
Text Solution
|
- Fig. shows a horzontal cylindrical container of length 30 cm, which is...
Text Solution
|
- As adiabatic vessel contains n(1) = 3 mole of diatomic gas. Moment of ...
Text Solution
|
- Figure. Shows an ideal gas changing its state A to state C by two diff...
Text Solution
|
- Fig. shows a process ABCA performed on 1 mole of an ideal gas. Find th...
Text Solution
|
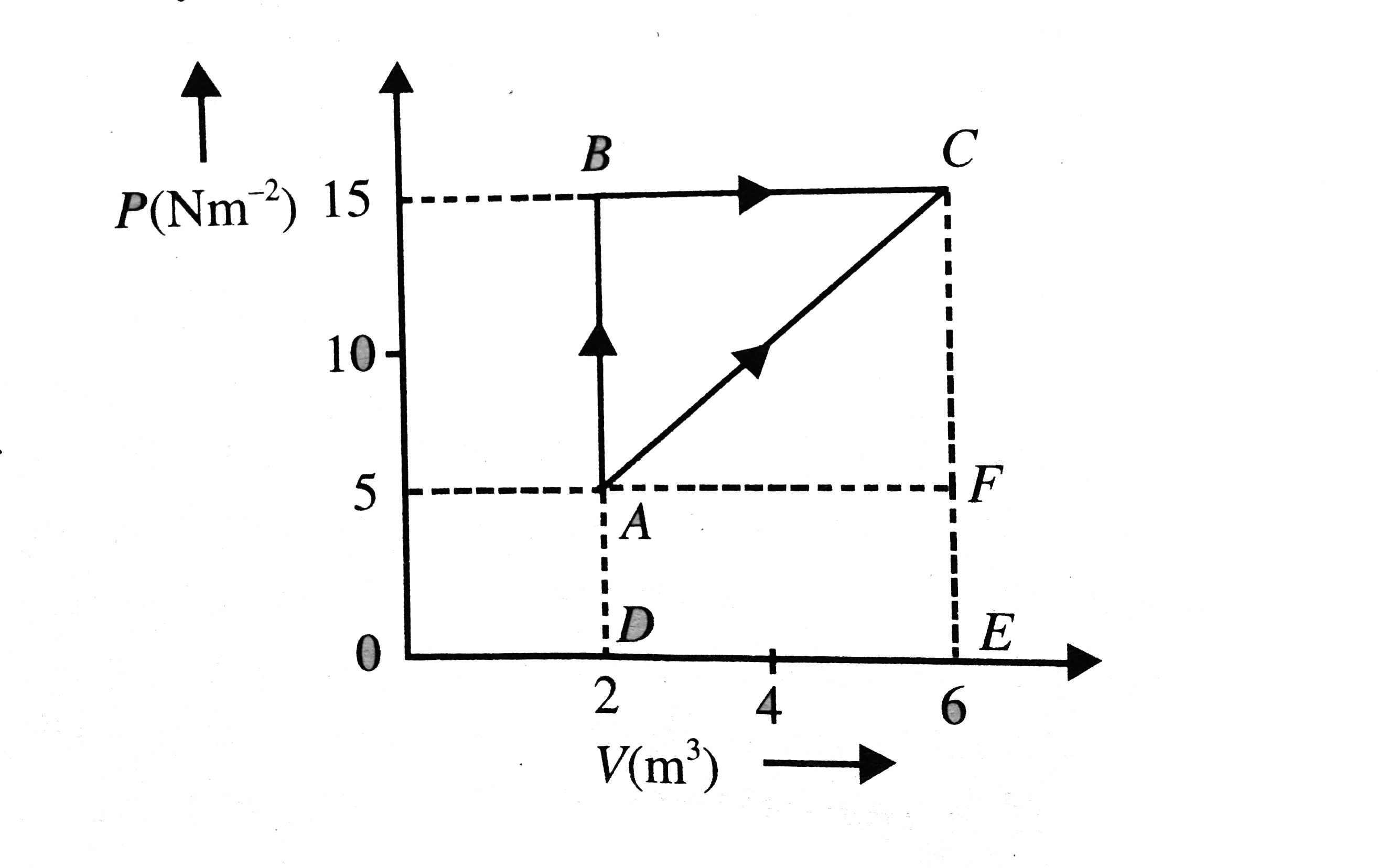
- Calculate the heat abosrbed by a system in going through the cyclic pr...
Text Solution
|
- An ideal gas has a volume 0.3 m^(3) at 150 k Pa. It is confined by a s...
Text Solution
|
- One mole of an ideal monatomic gas undergoes the process p=alphaT^(1//...
Text Solution
|
- Two moles of monatomic ideal gas is taken through a cyclic process sho...
Text Solution
|
- One mole of a gas is enclosed in a cylinde in a cyclinder and occupies...
Text Solution
|