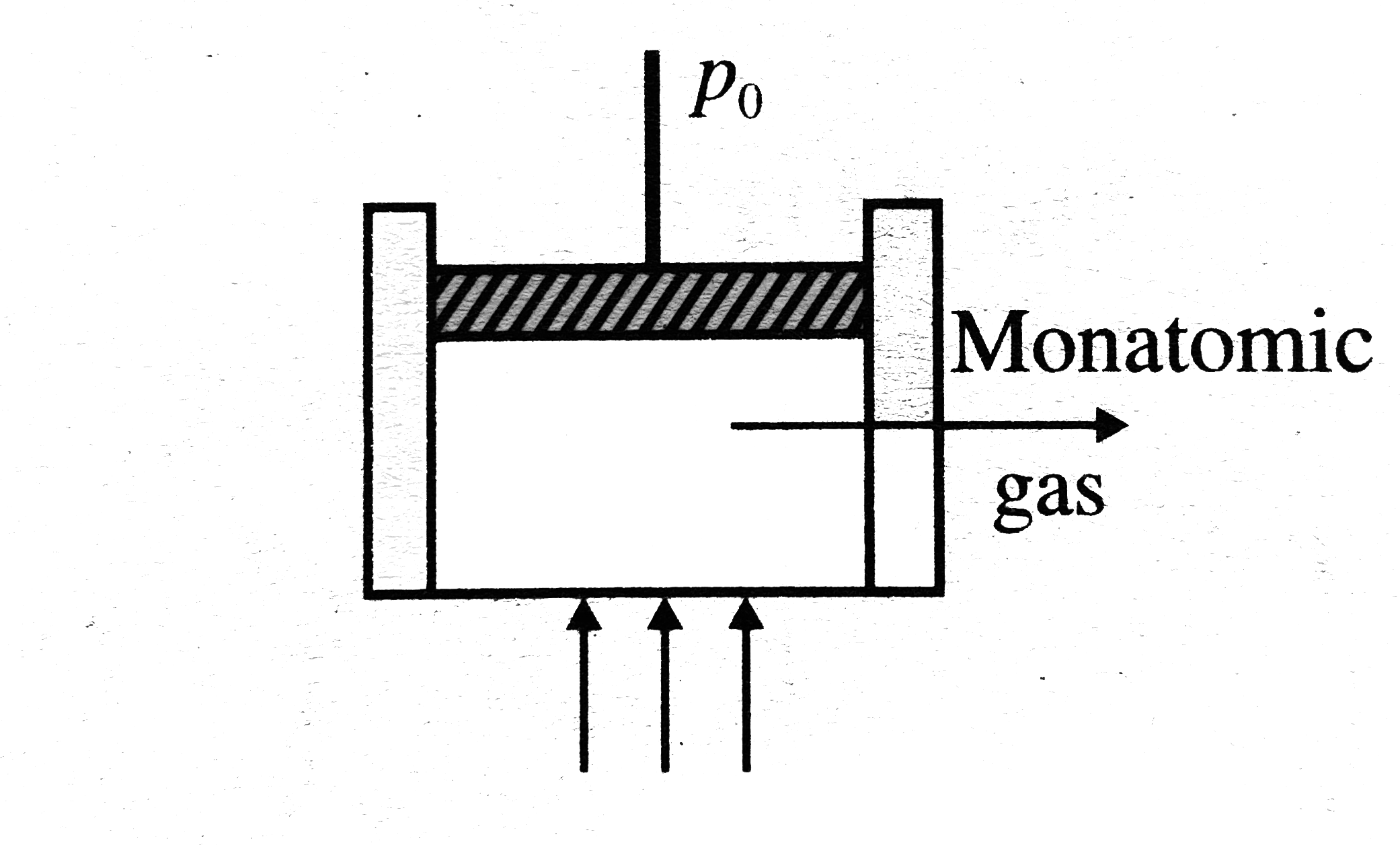
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Multiple Corrects|29 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Assertion-Reasoning|6 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Subjective|22 VideosKINETIC THEORY OF GASES
CENGAGE PHYSICS|Exercise Compression|2 VideosLINEAR AND ANGULAR SIMPLE HARMONIC MOTION
CENGAGE PHYSICS|Exercise Multiple Correct Answer Type|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS-Single Correct
- A cyclic process ABCA is shown in the V-T diagram process on the P-V
Text Solution
|
- One mole of a diatomic gas undergoes a process P = P(0)//[1 + (V//V(0)...
Text Solution
|
- If 50 cal of heat is supplied to the system containing 2 mol of an ide...
Text Solution
|
- In the given elliptical P-V diagram
Text Solution
|
- Three processes compose a thermodynamic cycle shown in the accompanyin...
Text Solution
|
- When an ideal gas is taken from state a to b, along a path acb, 84 kJ ...
Text Solution
|
- A sample of an ideal gas is taken through the cyclic-process ABCA show...
Text Solution
|
- Variation of internal energy with density of 1 "mole" of monatomic gas...
Text Solution
|
- One mole of an ideal gas is taken along the process in which PV' = co...
Text Solution
|
- A vessel of volume 20 L contains a mixture o hydrogen and helium at te...
Text Solution
|
- A cylinder of ideal gas is closed by an 8kg movable piston of area 60c...
Text Solution
|
- A certain ideal gas undergoes a polytropic process PV^(n) = constant s...
Text Solution
|
- A cylindrical chamber A of uniform cross section is divided into two p...
Text Solution
|
- An ideal gas (1 mol, monatomic) is in the intial state P (see Fig.) on...
Text Solution
|
- Figure shows an isochore, an isotherm, an adiabatic and two isobars of...
Text Solution
|
- A stationary cylinder of oxygent used in a hospital has the following ...
Text Solution
|
- A sample of ideal gas is expanded to twice its original volume of 1.00...
Text Solution
|
- One mole of air (C(V) = 5R//2) is confined at atmospheric pressure in ...
Text Solution
|
- A thermodynamic process is shown in Fig. The pressures and volumes cor...
Text Solution
|
- Carbon monoxide is carried around a closed cyclic processes abc, in wh...
Text Solution
|