A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Assertion-Reasoning|6 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Comprehension|56 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Single Correct|140 VideosKINETIC THEORY OF GASES
CENGAGE PHYSICS|Exercise Compression|2 VideosLINEAR AND ANGULAR SIMPLE HARMONIC MOTION
CENGAGE PHYSICS|Exercise Multiple Correct Answer Type|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS-Multiple Corrects
- Select the correct alternatives for an ideal gas :
Text Solution
|
- Refer to figure DeltaU(1) and DeltaU(2) be the changes in internal ene...
Text Solution
|
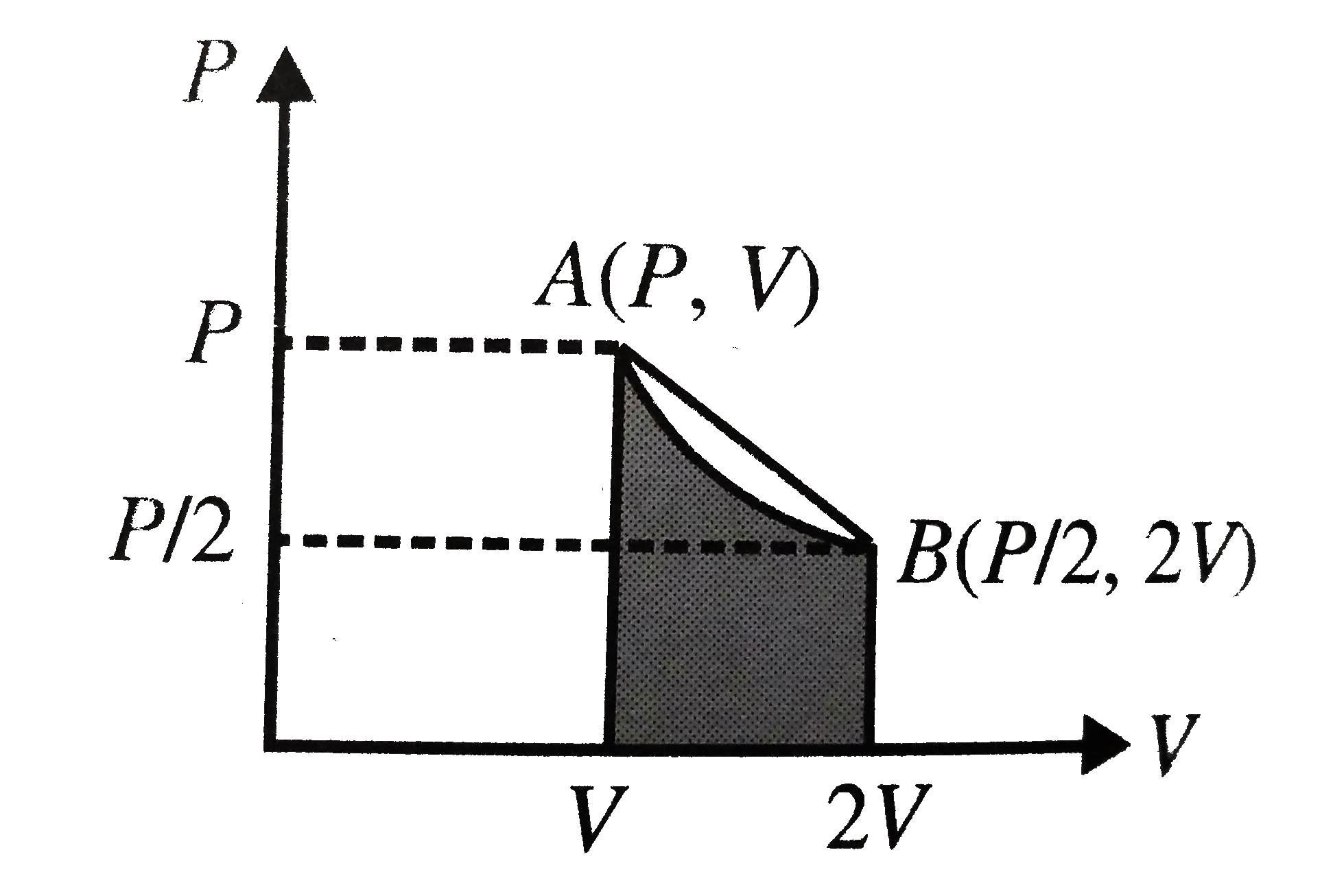
- An ideal gas is taken from the state A (pressure p, volume V) to the s...
Text Solution
|
- A thermally insulated chamber of volume 2V(0) is divided by a friction...
Text Solution
|
- In the arrangement shown in Fig. gas is thermally insulated. An ideal ...
Text Solution
|
- A gas undergoes change in its state from position A to position B via ...
Text Solution
|
- An ideal gas undergoes a thermodynamic cycle as shown in Fig. Which of...
Text Solution
|
- At ordinary temperatures, the molecules of an ideal gas have only tran...
Text Solution
|
- The molar heat capacity for an ideal gas cannot
Text Solution
|
- A closed vessel contains a mixture of two diatomic gases A and B. Mola...
Text Solution
|
- An ideal gas undergoes a thermodynamic cycle as shown in Fig. Which of...
Text Solution
|
- Which the following statements are correct ?
Text Solution
|
- Figure. Shows the P-V diagram for a Carnot cycle. In this diagram
Text Solution
|
- Figure shows an indicator diagram. During path 1-2-3, 100 cal is given...
Text Solution
|
- One mole of an ideal monatomic gas has initial temperature T(0), is ma...
Text Solution
|
- P - V diagram of a cyclic process ABCA is as shown in Fig. Choose the ...
Text Solution
|
- During the process AB of an ideal gas
Text Solution
|
- Temperature versus pressure graph of an ideal gas is shown in Fig. Dur...
Text Solution
|
- An ideal gas undergoes the cyclic process shown in a graph below :
Text Solution
|
- The indicator diagram for two processes 1 and 2 carrying on an ideal g...
Text Solution
|