A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Comprehension|56 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Interger|11 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Multiple Corrects|29 VideosKINETIC THEORY OF GASES
CENGAGE PHYSICS|Exercise Compression|2 VideosLINEAR AND ANGULAR SIMPLE HARMONIC MOTION
CENGAGE PHYSICS|Exercise Multiple Correct Answer Type|9 Videos
CENGAGE PHYSICS-KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS-Assertion-Reasoning
- Statement I: A quasi -static process is so called bacause it is a sud...
Text Solution
|
- Statement I: The work done on an ideal gas in changing its volume from...
Text Solution
|
- Statement I: When an ideal gas is taken from a given thermodynamics st...
Text Solution
|
- Statement I: The specific heat of a gas in an adiabatic process is zw...
Text Solution
|
- StatementI: Work done by a gas in isothermal expansion is more than th...
Text Solution
|
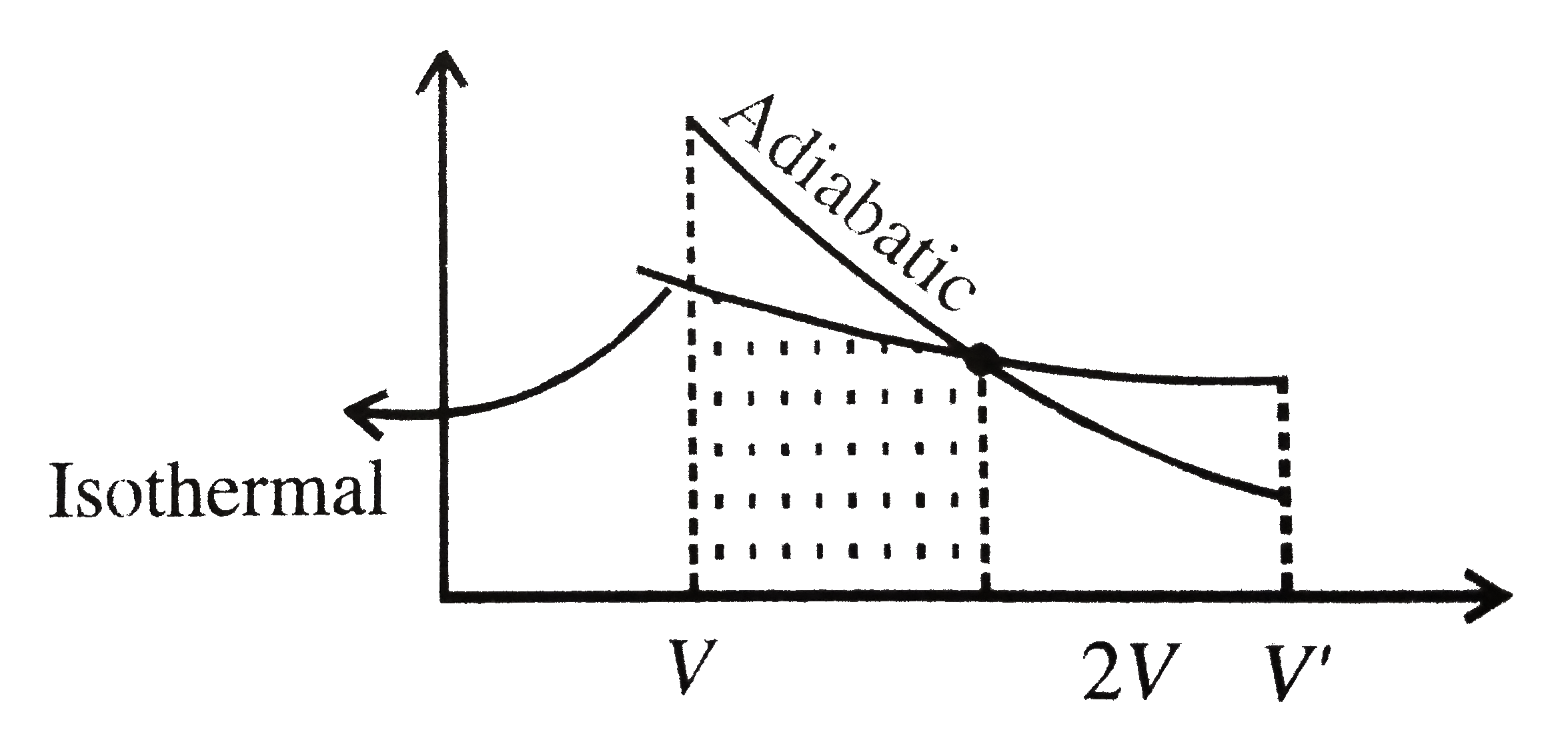
- StatementI: A gas is expanded from a volume V to 2V, first through adi...
Text Solution
|