A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Interger|11 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS|Exercise Assertion-Reasoning|6 VideosKINETIC THEORY OF GASES
CENGAGE PHYSICS|Exercise Compression|2 VideosLINEAR AND ANGULAR SIMPLE HARMONIC MOTION
CENGAGE PHYSICS|Exercise Multiple Correct Answer Type|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS-Comprehension
- A process in which work perfomed by an ideal gas is proportional to th...
Text Solution
|
- A process in which work perfomed by an ideal gas is proportional to th...
Text Solution
|
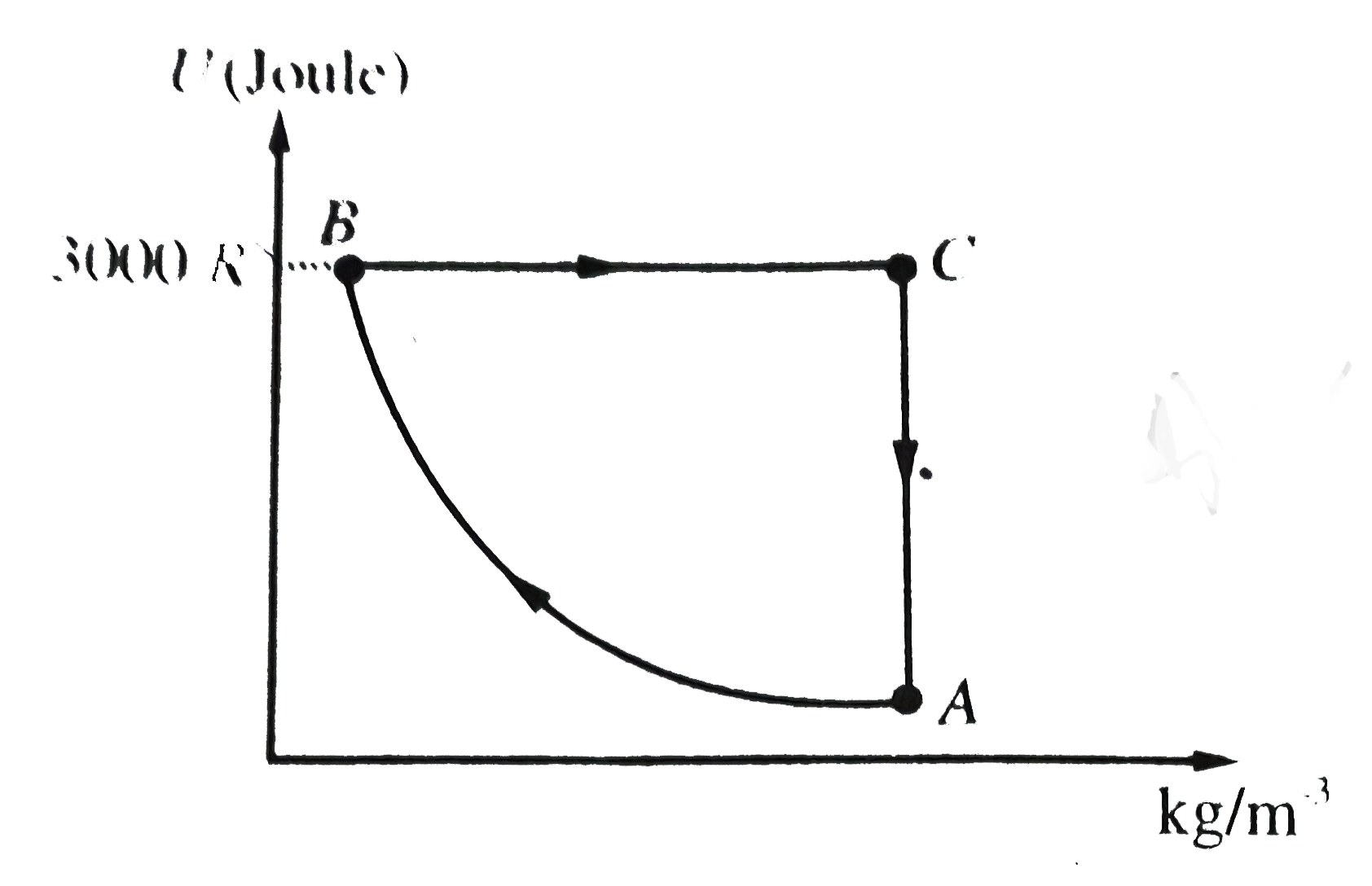
- Figure, shows the variation of potential energy (U) of 2 mol of Argon ...
Text Solution
|
- Figure, shows the variation of potential energy (U) of 2 mol of Argon ...
Text Solution
|
- Figure, shows the variation of potential energy (U) of 2 mol of Argon ...
Text Solution
|
- One mole of an ideal gas has an interal energy given by U=U(0)+2PV , w...
Text Solution
|
- One mole of an ideal gas has an interal energy given by U=U(0)+2PV , w...
Text Solution
|
- One mole of an ideal gas has an interal energy given by U=U(0)+2PV , w...
Text Solution
|
- An ideal diatomic gas is expanded so that the amount of heat transferr...
Text Solution
|
- An ideal diatomic gas is expanded so that the amount of heat transferr...
Text Solution
|
- An ideal diatomic gas is expanded so that the amount of heat transferr...
Text Solution
|
- A cylinder containing an ideal gas ( see figure ) and closed by a mova...
Text Solution
|
- A cylinder containing an ideal gas ( see figure ) and closed by a mova...
Text Solution
|
- A cylinder containing an ideal gas ( see figure ) and closed by a mova...
Text Solution
|
- A reversible heat engine carries 1 mol of an ideal monatomic gas aroun...
Text Solution
|
- A reversible heat engine carries 1 mol of an ideal monatomic gas aroun...
Text Solution
|
- A reversible heat engine carries 1 mol of an ideal monatomic gas aroun...
Text Solution
|
- A monatomic ideal gas undergoes the shown cyclic process in which path...
Text Solution
|
- A monatomic ideal gas undergoes the shown cyclic process in which path...
Text Solution
|
- A gas take part in two thermal processes in which it is heated from th...
Text Solution
|