Let `p` be the pressure in equilibrium, then
`pA=p_(0)A-Mg`
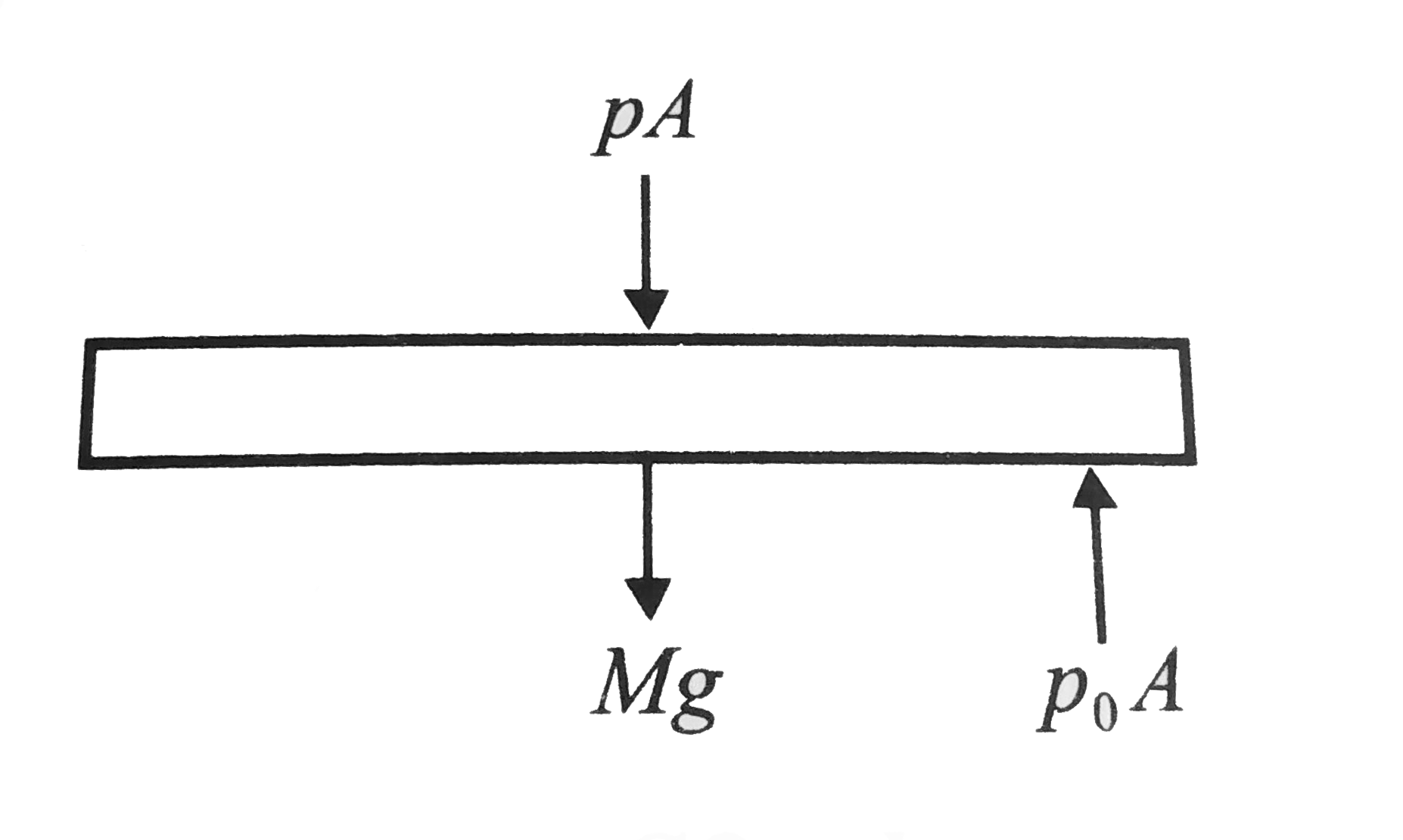
`p=p_(0)-(Mg)/(A)=p_(0)-(Mg)/(piR^(2))`
Applying `p_(1)V_(1)=p_(2)V_(2)`
`L'=(2p_(0)L)/(p)=((p_(0))/(p_(0)-(Mg)/(piR^(2))))(2L)`
`=((p_(0)piR^(2))/(piR^(2)p_(0)-Mg))(2L)`
Therefore, option `(d)` is correct.