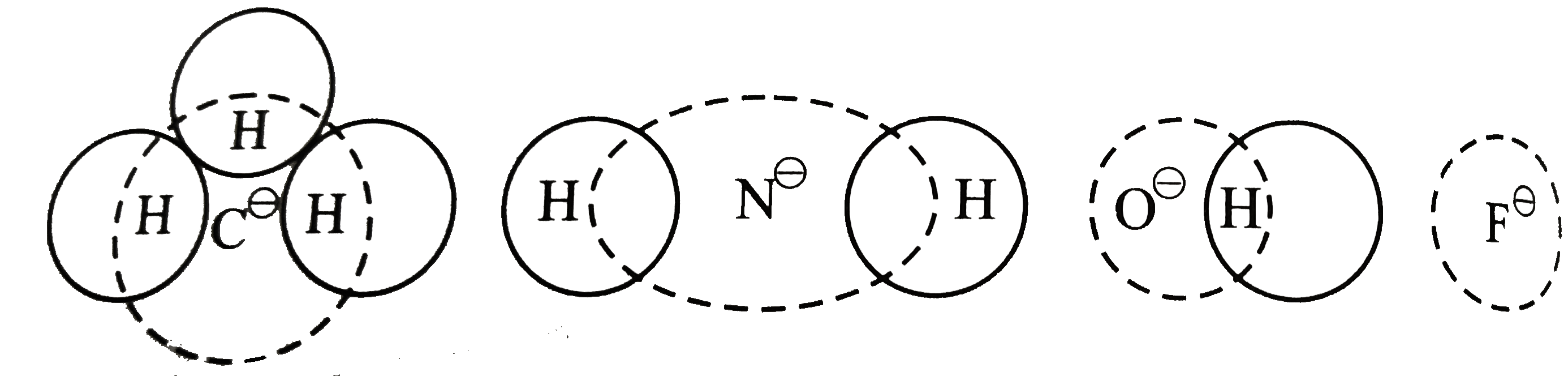Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Solved Examples|34 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 1.1|14 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|9 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise Assertion Reasoning Type|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY-Exercises (Archives )Subjective
- Give the decreasing order of acidic strength of the following : (a) ...
Text Solution
|
- Arrange the following in the given order (a) Decreasing ionic size, ...
Text Solution
|
- The IE(1) of C atom is greater than that of boron (B) atom, whereas th...
Text Solution
|
- Arrange the following as stated: Increasing order of ionic size N^(3...
Text Solution
|
- Arrange the following ions in order of their decreasing ionic radii. ...
Text Solution
|