a. `O^(-2) gt F^() gt Na^(o+) gt Mg^(2+)`

Smaller the value of `Z/bar(e)`, larger is the size.
Hence the order is as given above.
b.
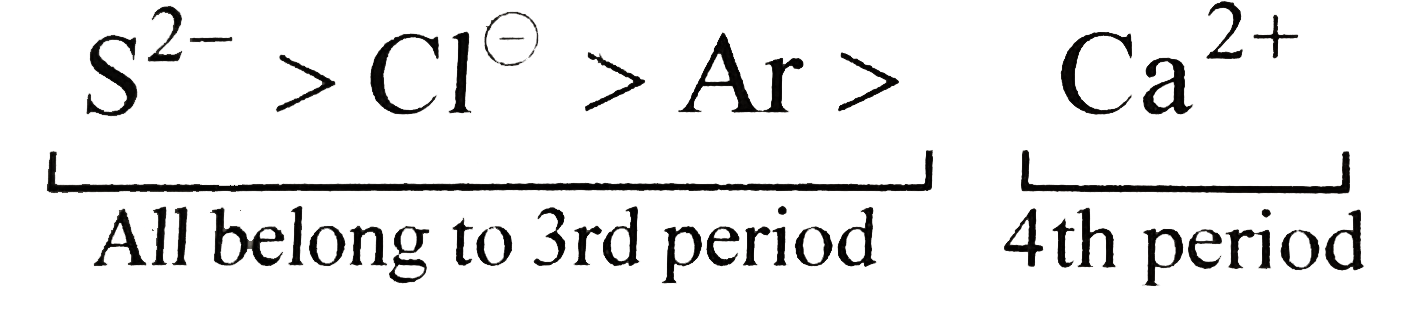
, `(("All species are"),("isoelectronic"))`
i. Size of element decreases along the same period `(rarr)` (due to greater electron-electron repulsion).
ii. Size of Ca (4th period) gt size of Ar (3rd period), but the size of Ar gt size of `Ca^(+)`. Therefore, Size of dinegative ion gt size of mononegative ion gt noble gas (of the same period) gt size of dipositive cation.
hence the order is as given above.
c. `N^(3-) gt O^(2-) gt F^() gt Na^(o+) gt Mg^(2+)` (All species are isoelectronic)
`|{:(Z,N=7,O=8,F=9,Na=11,Mg=12),(e^(-),7+3=10,8+2=10,9+1=10,11-1=10,12-2=10):}|`
Same explanation as in parts (a) and (b) above
d. `(Se)/("4th Period") gt (S)/("3rd Period") gt (C)/("2nd Period") gt (O)/("2nd Period")`
size of atom increases down the group because of addtion of new shell (or increase in principal quantum number n).
Size of atm decreases along the period `(rarr)`, i.e. decreases from C to O.
e. `(Ni gt)/("3rd Period")(Li gt )/("2nd Period")(Be gt B)/("2nd Period")`
Same explanation as in part (d) above.
f. `Li^(o+) gt Na^(o+) gt K^(o+) gt Rb^(o+) gt Cs^(o+)` (in aqueous solution)
The ions in solution are present as hydrate ions. The smaller the size of the ion, greater is the charge density and hence greater is the extent of hydration. So, the size of hydrated ions becomes larger for the smaller sized ion and vice versa.
g. `Na^(o+) gt Mg^(2+) gt Si^(4+) gt Cl^(7+)` (All species are isoelectronic)
`|{:(Z,Na=11,Mg=12,Si=14,Cl=17,),(bar(e),11-1=10,12-2=10,14-4=10,17-7=10,):}|`
Smaller the charge on the cation, larger is the size and vice versa.
h. `H^(ɵ) gt Li gt H^(o+)` (The species are not isoelectronic)
`|{:(Z,H=1,Li=3,H=1,),(bar(e),1+1=2,3+0=3,1-1=0,),((Z)/(e),(1)/(2) = 0.5,(3)/(8) = 1.0,-,):}|`
Smaller the value of `(Z)/(e^(-))`, larger is the size.
i. `O^(2-) gt F^(ɵ) gt Li^(o+) gt B^(3+)` (All species are not isoelectronic but all of them belong to the same 2nd period)
`|{:(Z,O=8,F=9,Li=3,B=5),(bar(e),8+2=10,9+1=10,3-1=2,5-3=2):}|`
Higher the `-ve` charge, larger is the size and higher the `+v` charge, smaller is the size of an ion j.
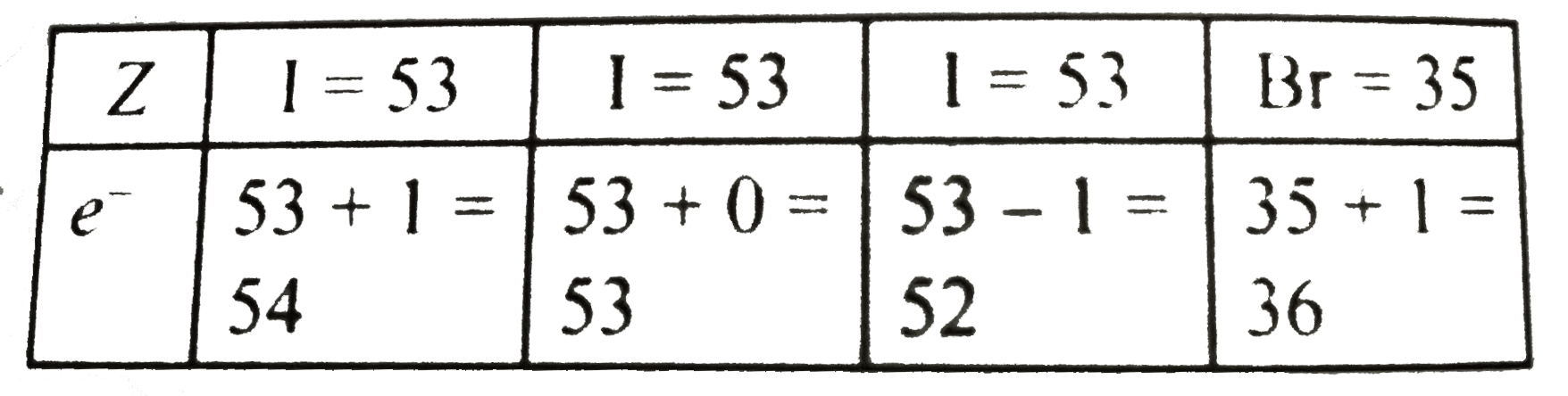
, [All the species are not-isoelectronic]
`|{:(Z,I=53,I=53,I=53,Br=35,),(e^(-),53+1=54,53+0=53,53-1=52,35+1=36,):}|`
Same explanation as in (a) and (b) above.
k. `I^(ɵ) gt I gt I^(o+)` [Same explanation as in (j)]
I. `K^(o+) gt Ca^(2+) gt Ti^(3+) gt Ti^(4+)` [Same explanation as in (g)]
m. `(Z "for" Ce = 58, Sn = 60, Yb = 70 "and" Lu = 71)`
`|("Group",1,2,-,13,14,15,16,17,18),("Element",Na,Mg,,Al,Si,P,S,Cl,Ar)|`
In lanthanides, the size decreases from `La` to `Lu (Z = 57 "to" 71)` due to lanthanide contration. Although `Sn` belongs to the 5th period but its size is larger than lanthanides.
n. `O^(2-) gt F^(ɵ) gt O gt F`
(The species are not isoelectronic)
[Same explanationas in parts (a) and (b) above]
o. `Br gt Ar gt Ca^(2+) gt Mg^(2+)`

 , `(("All species are"),("isoelectronic"))`
, `(("All species are"),("isoelectronic"))` 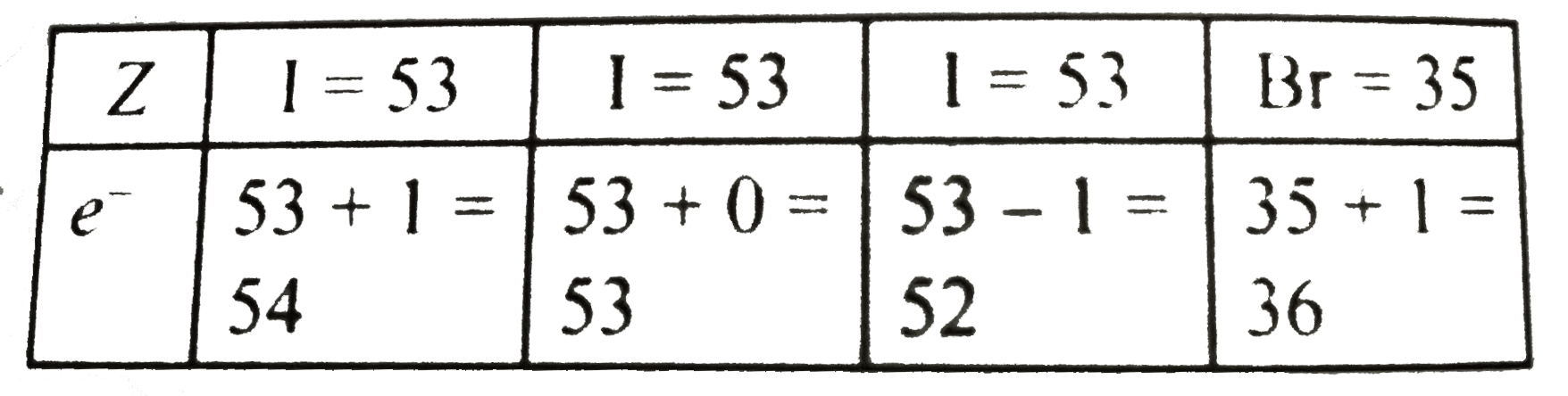 , [All the species are not-isoelectronic]
, [All the species are not-isoelectronic]