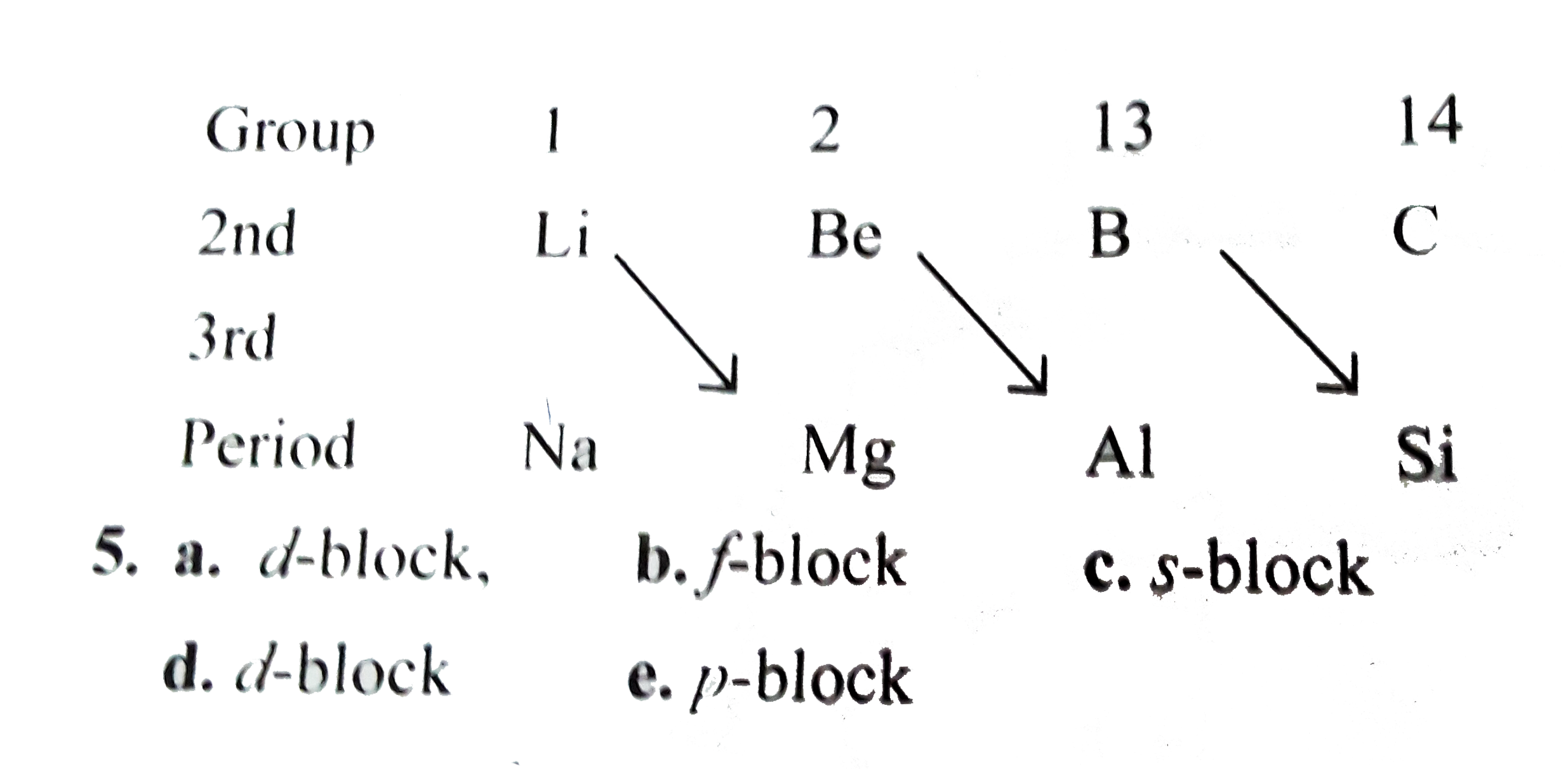Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 1.1 (Very Short)|22 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 1.1 (Objective)|12 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Solved Examples|34 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|9 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise Assertion Reasoning Type|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY-Ex 1.1
- 3d-, 4d- and 5d- series consists of 10 elements each? Explain.
Text Solution
|
- Why the f-block elements are called inner transition elements?
Text Solution
|
- Transition elements show horizontal as well as vertical relationship. ...
Text Solution
|
- Be and Al are placed in different periods and groups but they show the...
Text Solution
|
- The outer electronic configuration of some elements are given below: ...
Text Solution
|
- Arrange the following elements in decreasing order of metallic charact...
Text Solution
|
- Name the species that will be isoelectronic with the following atoms o...
Text Solution
|
- Which of the following pairs would have a smaller size. Explain. (a)...
Text Solution
|
- Arrange the following ions in order of their decreasing ionic radii. ...
Text Solution
|
- What property did Mendelev use to classify the elements in his perodic...
Text Solution
|
- Elements with Z =107, 108 and 109 have been made recently. Indicate t...
Text Solution
|
- Why Zn, Cd and Hg are not considered as typical transitions elements ?
Text Solution
|
- Why Cu, Ag and Au are transition elementsm although they have complete...
Text Solution
|
- Stability Of Completely Filled And Half Filled Orbitals
Text Solution
|