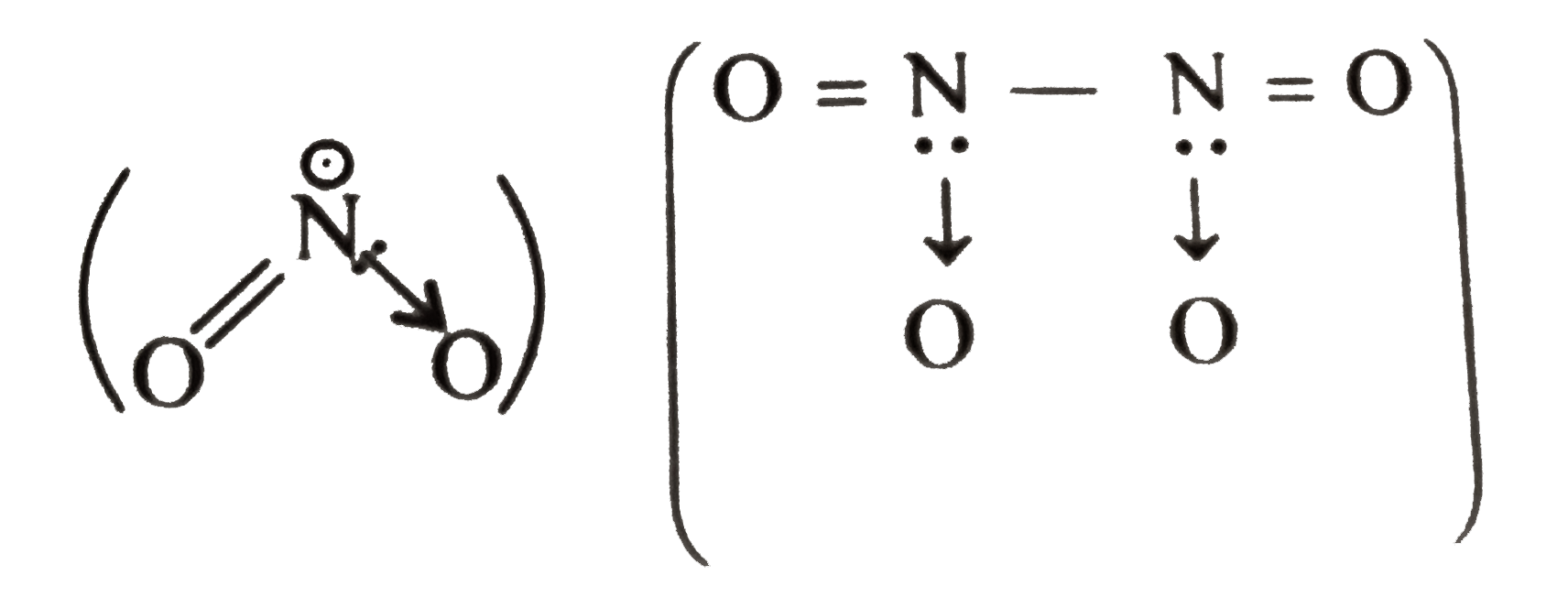A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 1.3|13 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 1.3 (Objective)|8 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 1.2|1 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|9 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise Assertion Reasoning Type|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY-Ex 1.2(Objective)
- The correct order of hydration enthalpies of alkali metal ions is:
Text Solution
|
- Inert pair effect is shown by
Text Solution
|
- Which is//are ampoteric oxide ?
Text Solution
|
- EA is positive when
Text Solution
|
- Which has the maximum covalent character?
Text Solution
|
- In which sovalent KBr has maximum solubility?
Text Solution
|
- Lattice energy of BeCO(3)(I), MgCO(3)(II) and CaCO(3)(III) is in order...
Text Solution
|
- NO(2) and N(2)O(4) are two forms of nitrogen dioxide. One exists in ga...
Text Solution
|
- Magnetic moment of V(Z = 23),Cr(Z = 24),and Mn(Z= 25) are x,y,z repe...
Text Solution
|
- Solubility of groups 1 and 2 fluorides increases down the group'. Whic...
Text Solution
|
- Which of the following molecule is theoretically not possible ?
Text Solution
|
- Which of the following triads have approximately equal size ?
Text Solution
|
- Which pair is different from the others ?
Text Solution
|
- Compound XY is predominantly ionic as X^(o+)Y^(ɵ) if
Text Solution
|
- (X), (Y), (Z) are elements in third short period. Oxide of (X) is ioni...
Text Solution
|
- Which of the correct order of size ? (O^(ɵ), O^(2-), F^(ɵ) and F ?)
Text Solution
|
- F has the highest electronegativity among the group 17 elements (i.e. ...
Text Solution
|
- The correct order of decreasing ionic character is
Text Solution
|
- The correct order of decreasing polarisability of ion is
Text Solution
|
- Which of the following has the smallest bond length ?
Text Solution
|