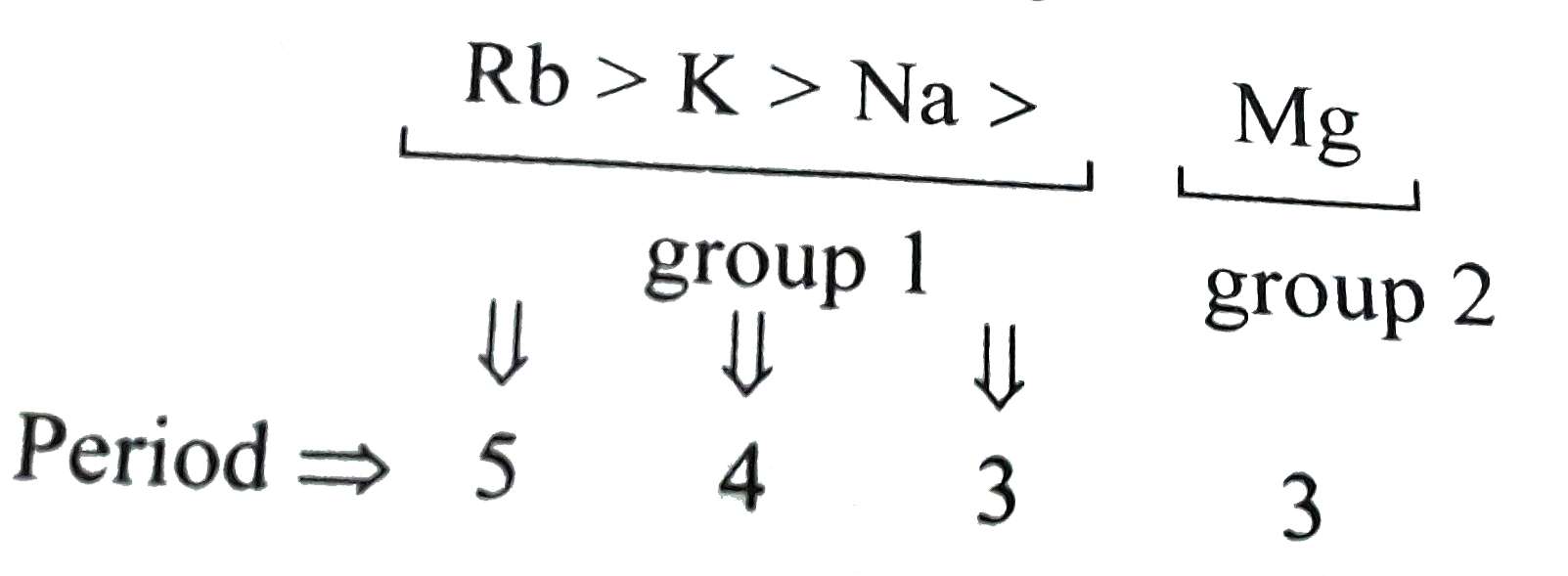A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct) Ionisation Energy (Ie)|31 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct) Isoelectronic Species|4 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercises (Singlecorrect) General Electronic Configuration And Periodicity|25 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|9 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise Assertion Reasoning Type|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY-Exercises (Singlecorrect) Atomic And Ionic Radii
- Which of the following has the largest ionic radius ?
Text Solution
|
- The size of species I, I^(+) and I^(ɵ) decrease in the order.
Text Solution
|
- Which one of the following is the smallest in size?
Text Solution
|
- Which of the following represent increasing order of size of 4th perio...
Text Solution
|
- Which of the following van der Waals radii is the largest ?
Text Solution
|
- The correct order of the size of Be, C, N, P and S is
Text Solution
|
- The correct order of the size of Be, C, F and Ne is
Text Solution
|
- The correct order of increasing radii are
Text Solution
|
- The correct arrangement of decreasing order of atomic radius among Na,...
Text Solution
|
- Which of the following pairs of elements have almost similar atomic ra...
Text Solution
|
- The radius of isoelectronic species
Text Solution
|
- Atomic radil of fluorine and neon in Angstrom units are respectively ...
Text Solution
|
- Anything that influences the valence electrons will affect the chemist...
Text Solution
|
- The size of isoelectronic species F^(ɵ), Ne, and Na^(o+) is affected b...
Text Solution
|
- Ionic radii of :
Text Solution
|