Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Solved Examples|36 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Ex 2 .2 Subjective (Intermolecular Forces And H-Bonding)|7 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Archives Subjective
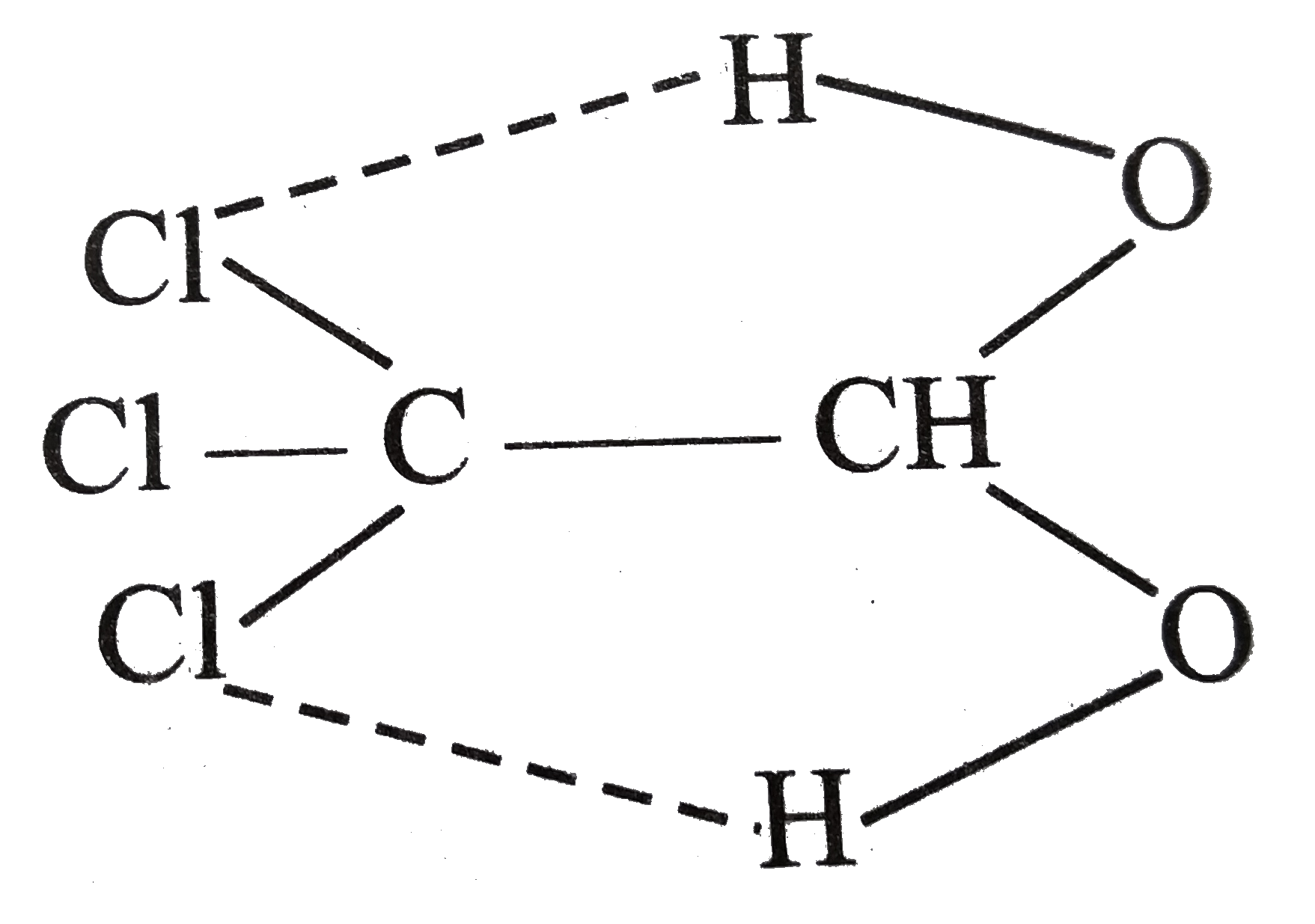
- Explain unusual stabillity of chorohybrate thugh a compound with two o...
Text Solution
|
- State four major physical properties that can be used to distinguish b...
Text Solution
|
- Write the Lewis dot structural formula for each of the following Also ...
Text Solution
|
- How many sigma bonds and pi bonds are present in a benzene molecules ?...
Text Solution
|
- Arrange the following as stated Increasing strenght of hybrogen bond...
Text Solution
|
- Given reasons in two or there sentences only for the following Hydroge...
Text Solution
|
- The dipole momnet of KCI is 3.336 xx 10^(-29) Cm which indicates that ...
Text Solution
|
- Explain the difference in the nature of bonding in LiF and LiI .
Text Solution
|
- Using the VSEPR theory, identify the type of hybisation and draw the s...
Text Solution
|
- Interpret the non-linear shape of H(2)S molecule and non-planar shape ...
Text Solution
|
- Write the MO electron distribution of O(2) Specify its bond order and ...
Text Solution
|
- Which one is more soluble in diethyl ether : anhydrous AlCl(3) or hydr...
Text Solution
|
- Using VSEPR theory draw the shape of PCI(5) and BrF(5) ?.
Text Solution
|
- Draw the shape of XeF(4) and OSF(4) according to VSEPR theory Show the...
Text Solution
|
- One the basic of ground electronic configuration, arrange the followin...
Text Solution
|
- Predict whether the following molecules are isostructural or not Justi...
Text Solution
|
 .
.