Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Ex 2 .2 Subjective (Intermolecular Forces And H-Bonding)|7 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Ex 2 .2 Subjective (Molecular Orbital Theory)|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Archives Subjective|15 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Solved Examples
- Construct a table comparing metals with non-metals in terms of (a) T...
Text Solution
|
- In which of the following compounds is the bonding essentially ionic i...
Text Solution
|
- Pure liquid H(2)SO(4) solidifies below 10.4^(@)C Neither the pure liqu...
Text Solution
|
- Write electron dot and line structure for (a) SeO(3)^(2-) ,(b) Li(3)...
Text Solution
|
- By completing the following structures, adding unshared e^(-) pairs wh...
Text Solution
|
- In each of the following paris select the species having the greater r...
Text Solution
|
- Distinguish between a polar bond and a polar molecule To which does th...
Text Solution
|
- The decreasing order of dipole moment of SO(2) gt NH(3) gt AsH(3) gt B...
Text Solution
|
- The single and multiple bond radii of some elements given in the follo...
Text Solution
|
- Arrange C-C,C=C and C-=C in order of (i) Decreasing bond energey (...
Text Solution
|
- The Pt-CI distance is 2.32 A in several crystaline compounds What is...
Text Solution
|
- The averge (C C) bond energy is 343 kJ mo1^(-1). What do you predict f...
Text Solution
|
- Compare the shapes of p-orbital and sp-hybrid orbital Which on has a g...
Text Solution
|
- Complete the following table (b) Which of the sets of hybridised orb...
Text Solution
|
- What is the number of molecular orbitals obtained by mixing of two ato...
Text Solution
|
- Compare and contrast the concepts of hybrid orbitals and molecular orb...
Text Solution
|
- State the bond order and indicate whether the species is paramagnetic ...
Text Solution
|
- Which properties of element depend on the electronic configuration of ...
Text Solution
|
- Select from each of the following gropus, the one which has the larges...
Text Solution
|
- Give the decreasing order of the property mentioned against each of th...
Text Solution
|
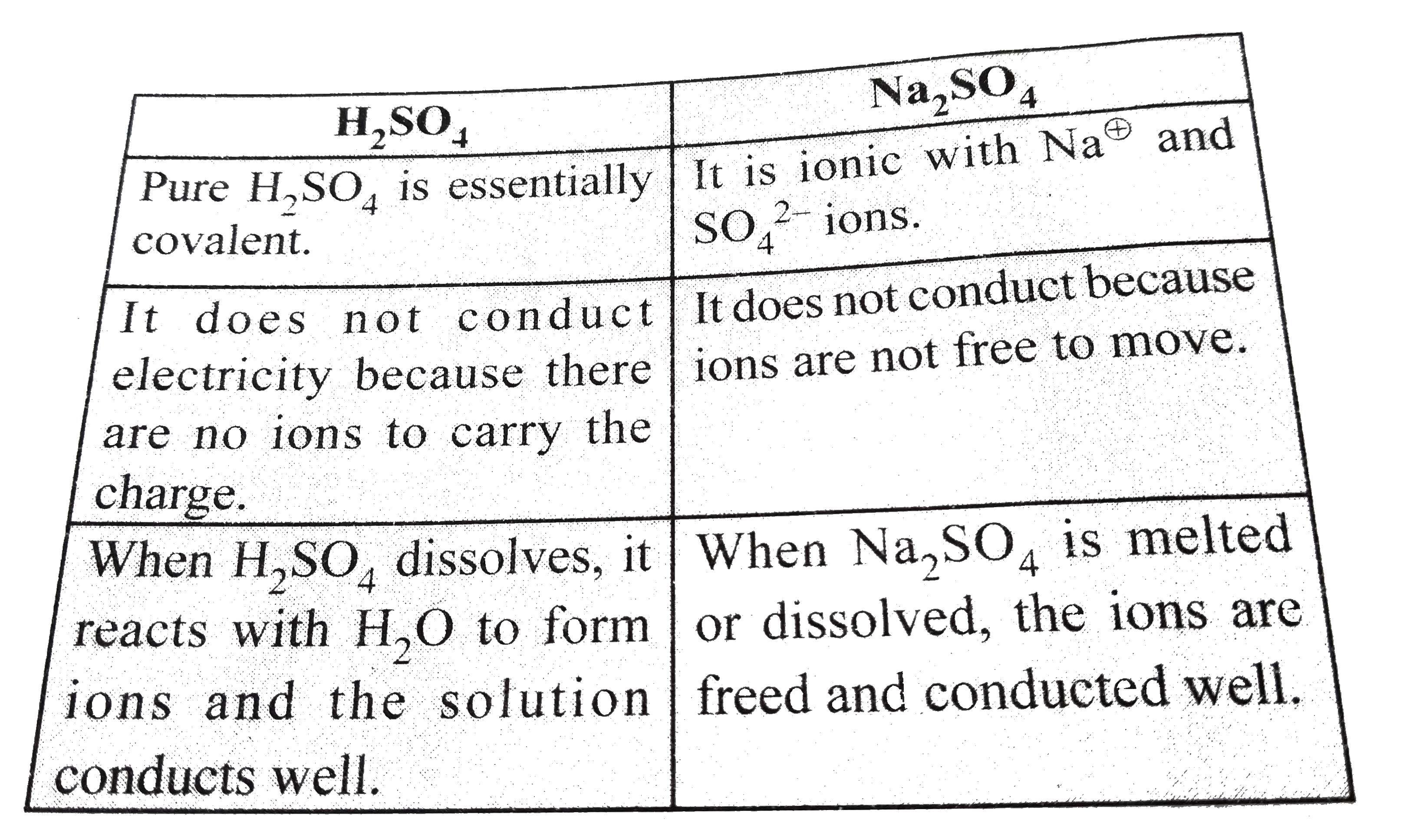 .
.