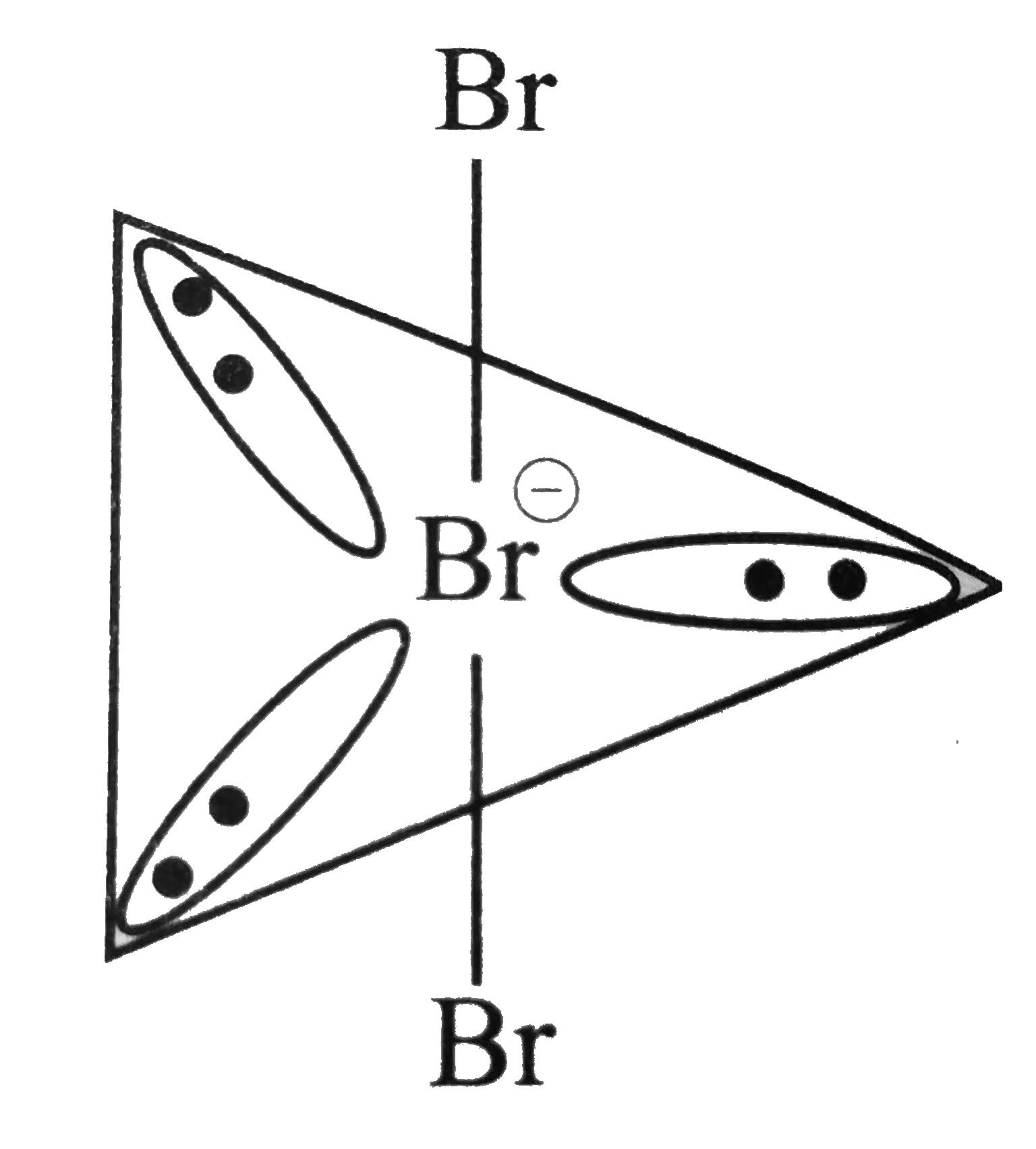
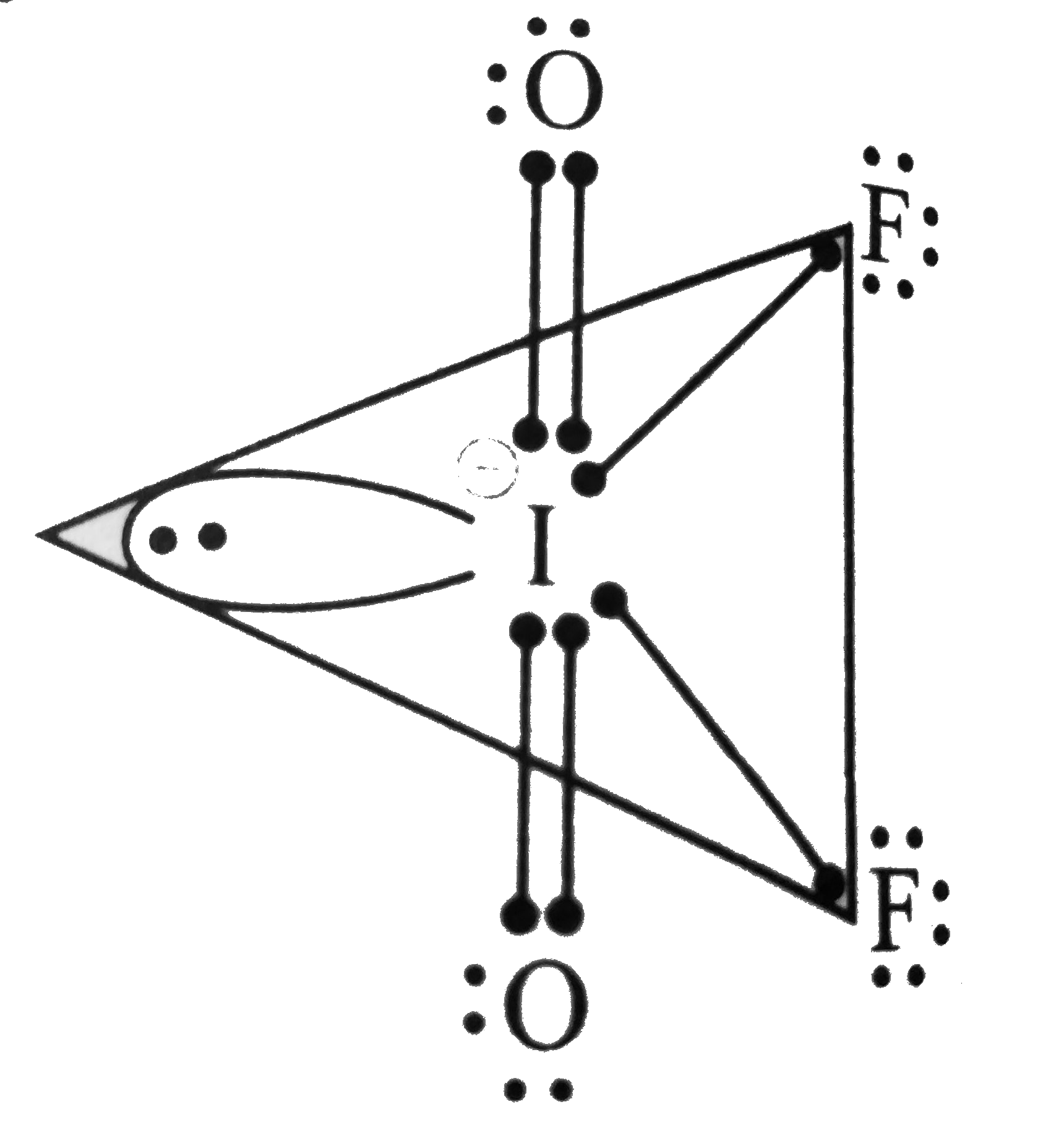
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Ex 2 .2 Subjective (Intermolecular Forces And H-Bonding)|7 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Ex 2 .2 Subjective (Molecular Orbital Theory)|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Archives Subjective|15 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Solved Examples
- State the bond order and indicate whether the species is paramagnetic ...
Text Solution
|
- Which properties of element depend on the electronic configuration of ...
Text Solution
|
- Select from each of the following gropus, the one which has the larges...
Text Solution
|
- Give the decreasing order of the property mentioned against each of th...
Text Solution
|
- Answer the following (a) How many sigma, pi non - bonding electrons ...
Text Solution
|
- Explain (a )Which d-orbital in involved in (i) sp^(3)d hybridisati...
Text Solution
|
- How do you account for the difference in melting points between (a) an...
Text Solution
|
- A plant virus was found to consist of uniform cylindrical particles 10...
Text Solution
|
- Calculate the I-I distance in each of the isomeric compounds C(2)H(2)I...
Text Solution
|
- Calculate the I-I distance in each of the three isomeric diiodobenzene...
Text Solution
|
- Enthalpic of hydrogenation of ethene (C(2)H(4)) and benzene (C(6)H(6))...
Text Solution
|
- Select the species which is best described to the right (a) CI(2),Br...
Text Solution
|
- The CI-O bond distance in CIO(4)^(Θ) is 144pm What do you conclude abo...
Text Solution
|
- Draw all geometrical isomers of PBr(2)CI(3) molecule State which isome...
Text Solution
|
- Write electron dot structures and describe the geometry of the followi...
Text Solution
|
- Reduce the hybridisation, geometry and shape of the following (i) CH...
Text Solution
|
- State the bond order and indicate whether the species is paramagnetic ...
Text Solution
|
- If the internuclear axis in the diatomic molecule AB is designated as ...
Text Solution
|
- Make a table giving (i) number of orbitals with a given energy (ii) ma...
Text Solution
|
- When 2s orbital overlaps with 2p(x) or 2p(y) orbital (assuming Z -axis...
Text Solution
|