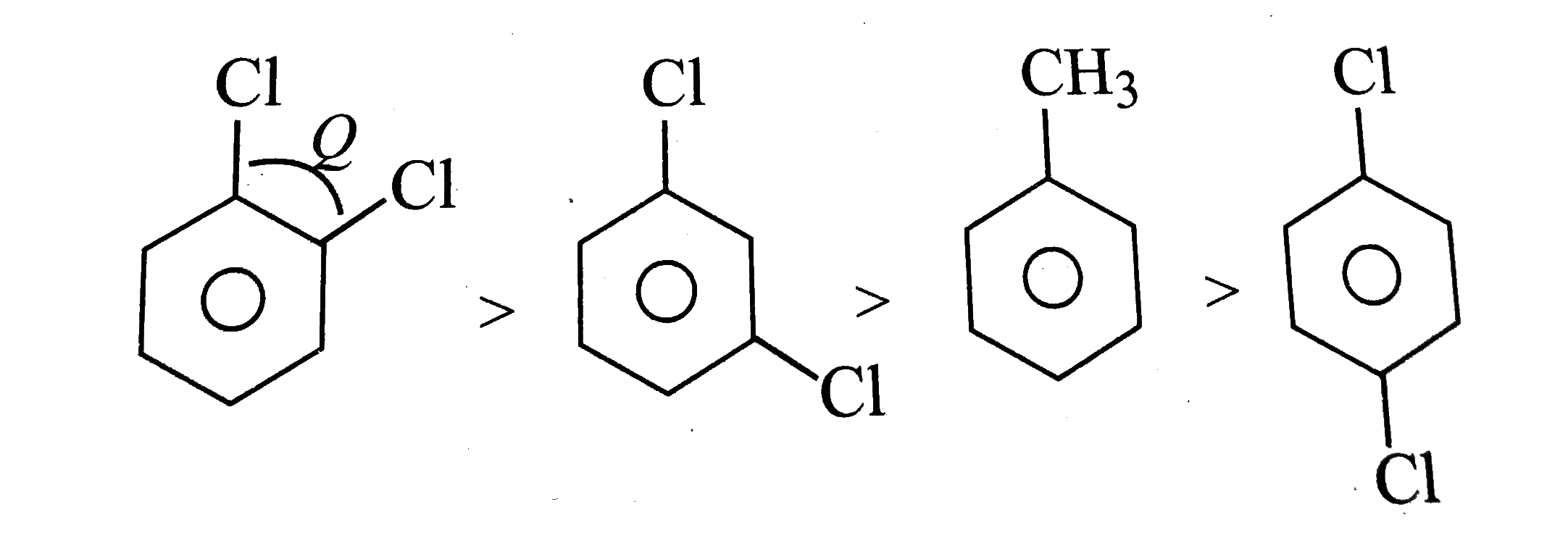
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Linked Comprehension|43 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct(Chemical Bonding )|7 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Ex 2 .2 Subjective (Molecular Orbital Theory)|4 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Ex 2 .2 Objective
- Arrange the following as directed (a) N(2),O(2),F(2),CI(2) (Decreasi...
Text Solution
|
- Expalin the following (a) The central C-C bond in Buta 1,3 diene is ...
Text Solution
|
- Arrange the following in decreasing order of dipole moment (a) Tolue...
Text Solution
|
- Predict the shape of the following Xenon compounds (a) XeO(3) (b) Xe...
Text Solution
|
- Considering X axis as the internuvlear axis, which out of the followin...
Text Solution
|
- Arrange the following types of interation in order of decreasing stabi...
Text Solution
|
- Arrange the following types of intermolecular forces in order of decre...
Text Solution
|
- Name the types of interaction or intermolecular forces of which potent...
Text Solution
|
- List properties of water that stem from H-bonding (b) Two two molecu...
Text Solution
|
- Perdict the order of decreasing boiling points of noble gases (b) Pr...
Text Solution
|
- Which of the following pairs is expected to exhibit H-bonding (a) CH...
Text Solution
|
- Give the decreasing order of melting points of the following NH(3),PH(...
Text Solution
|
- How many nodal planes are present in the following MO's (Taking Z-axis...
Text Solution
|
- Compare the relative stabilities and magnetic behaviour of the followi...
Text Solution
|
- Expalin (a) H(2)^(o+) and H(2)^(Θ) ions have same bond order but H(2...
Text Solution
|
- Which of the following species have same bond order and same shape (...
Text Solution
|
- Which of the following is soluble in water
Text Solution
|
- Which one among the following does not have the hybrogen bond ?
Text Solution
|
- The molecule having one unpaired electrons is .
Text Solution
|
- The H-bond is strongest in
Text Solution
|