A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct(Hydrogen Bonding)|3 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct(Bond Angle )|5 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct (Dipole Moment)|3 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Multiple Correct(Hybridisation)
- Which of the following are tetrahedral structures ? .
Text Solution
|
- Which among the following are isostructural ? .
Text Solution
|
- In which of the following molecules all the atoms lie in one plane ? .
Text Solution
|
- Which of the following have sp^(3) d hybridisation of the central atom...
Text Solution
|
- Which are the species in which central atom undergoes sp^(3) hybridisa...
Text Solution
|
- The pair od species having identical shapes for molecules of both spec...
Text Solution
|
- Which among the following is (are) having two lone pair of electrons o...
Text Solution
|
- The state of hybridisation of atoms in boric acid (H(3)BO(3)) is .
Text Solution
|
- Which of the following have sp^(3) d hybridisation ? .
Text Solution
|
- The hybridisation number of lone pair of electron and shape of I(3)^(Θ...
Text Solution
|
- Which of following is (are) correct for B and N in NH(3).BF(3) adduct ...
Text Solution
|
- Which of the following is not square planar ? .
Text Solution
|
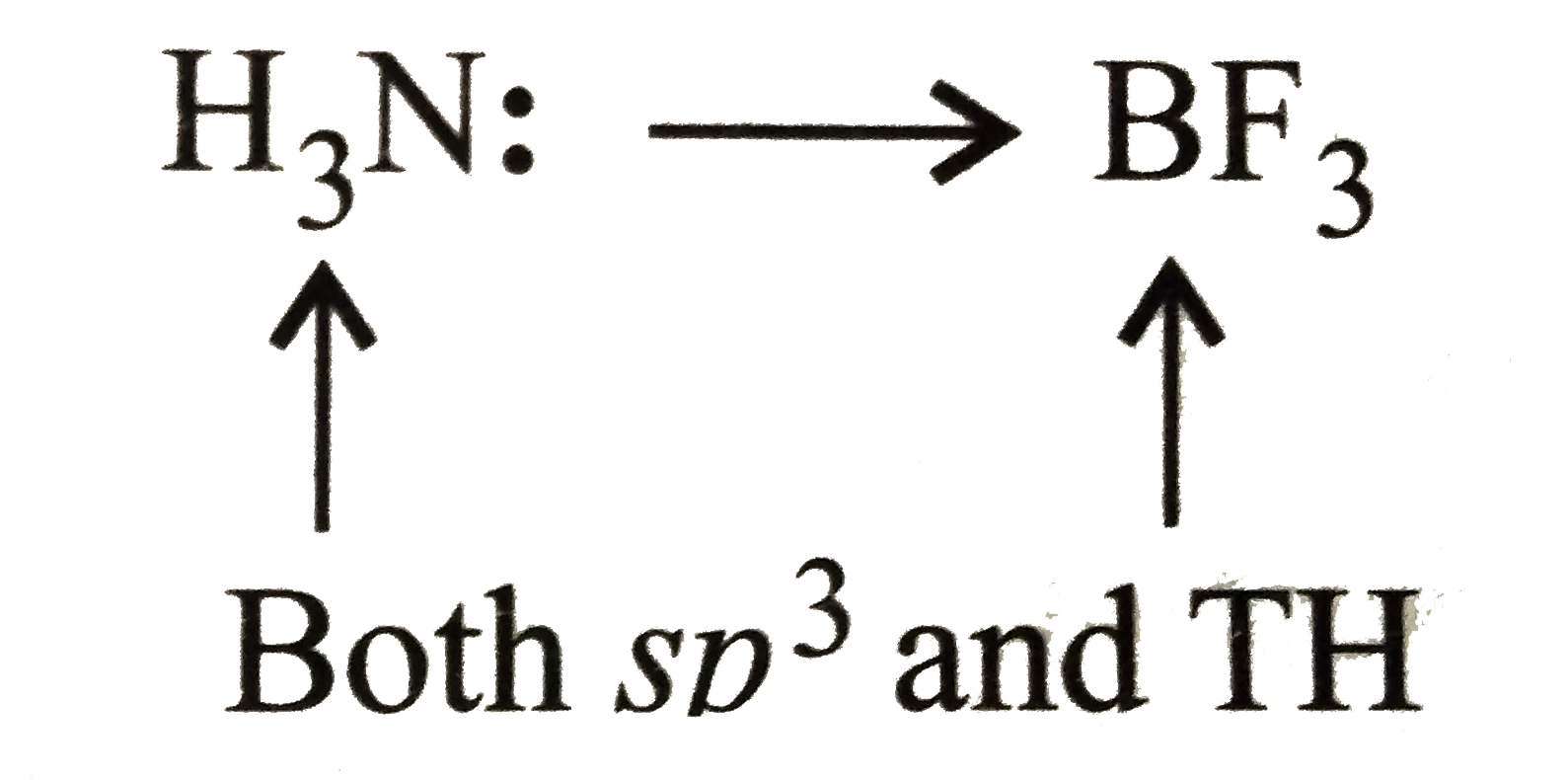 .
.