A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct (Miscellaneous)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Chemical Bonding)|46 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct(Bond Angle )|5 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Multiple Correct(Molecular Orbitaltheory (Mot))
- Which of the folowing have identical bond orders ? .
Text Solution
|
- Which of the following diatomic molecule //ions have same bond order ?...
Text Solution
|
- Which of the following species exhibits the diamagnetic behaviour ?
Text Solution
|
- Which of the following molecules has one unpiared electron in antibond...
Text Solution
|
- Which of the following show paramagnetism ? .
Text Solution
|
- Which of the following is (are) correct statements ? .
Text Solution
|
- Which of the following is (are) gerade (g) MO's ? .
Text Solution
|
- MO' s are formed by the overlap of A'O s Two AO's combine to form two ...
Text Solution
|
- Which of the following MO s have one nodal plane ? .
Text Solution
|
- Which of the following MO's have two nodal plane ? .
Text Solution
|
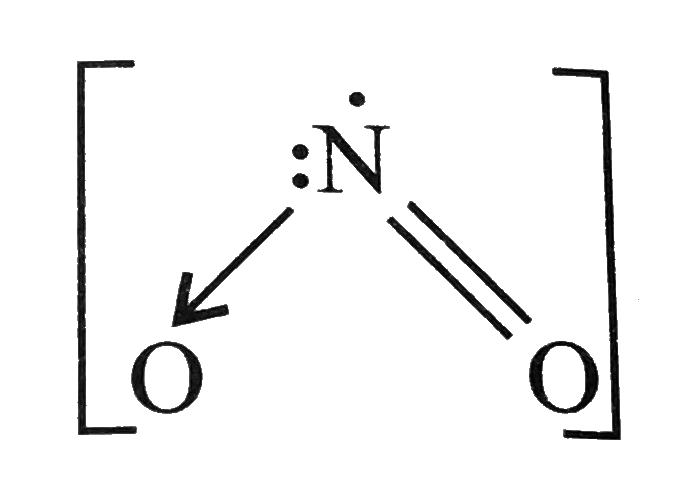
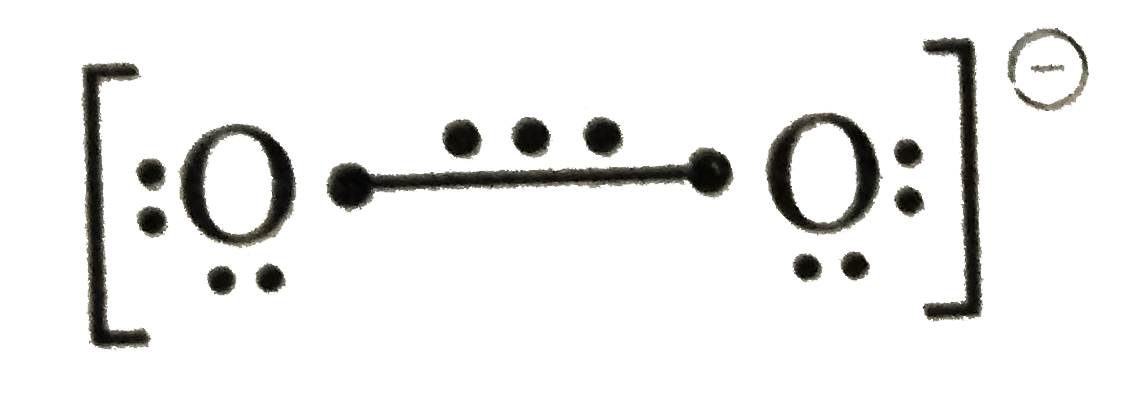 .
.