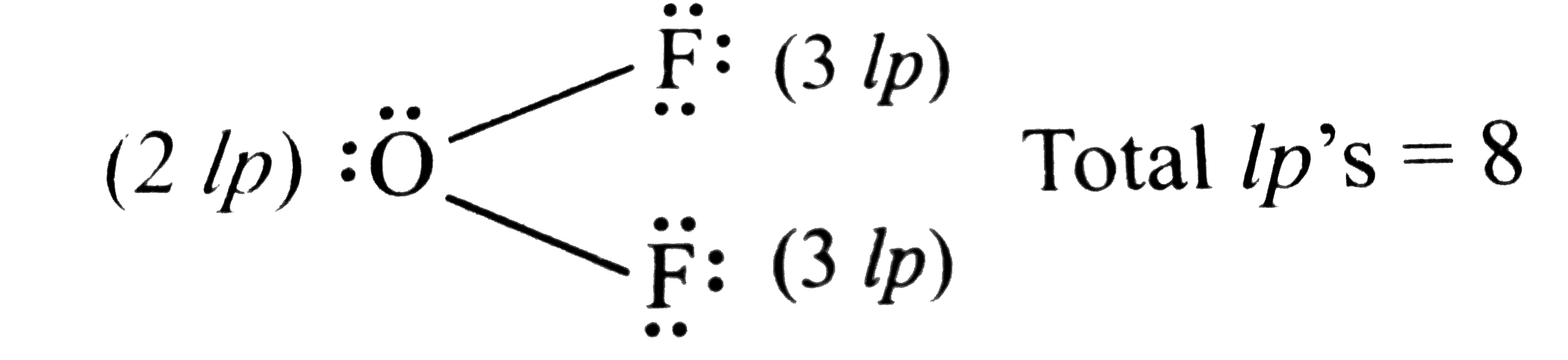Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Fill In The Blanks|20 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises True/False|20 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Assetion Reasoning|30 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Integer
- How many molecules among the following have zero dipole moment NH(3),B...
Text Solution
|
- Find the number of lone pairs of electrons preent in OF(2) .
Text Solution
|
- How many of the following compounds violate octet rule (i) BrF(5) (i...
Text Solution
|
- The number of hypervalent species among the following CIO(4)^(Theta)...
Text Solution
|
- The number of correct options is (a) 1^(Theta) gt Br^(Theta) gt CI^(...
Text Solution
|
- How many of the following compounds have sp^(3) hybridisation (i) SO...
Text Solution
|
- How many of the following compounds have (p pi-dpi) multiple bonds (...
Text Solution
|
- How many of the following oxides of nitrogen are paramangnetic ? (i)...
Text Solution
|
- How many of the following species have bond order of 2.5 ? N(2)^(o+)...
Text Solution
|
- The number of correct option is (a) P(2)O(5) gtZnO gtMgO gt Na(2)O(2...
Text Solution
|