Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Solved Examples|8 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Ex 3.1|4 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive|1 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives Subjective|28 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-HYDROGEN, WATER AND HYDROGEN PEROXIDE-Subjective Archive (Subjective)
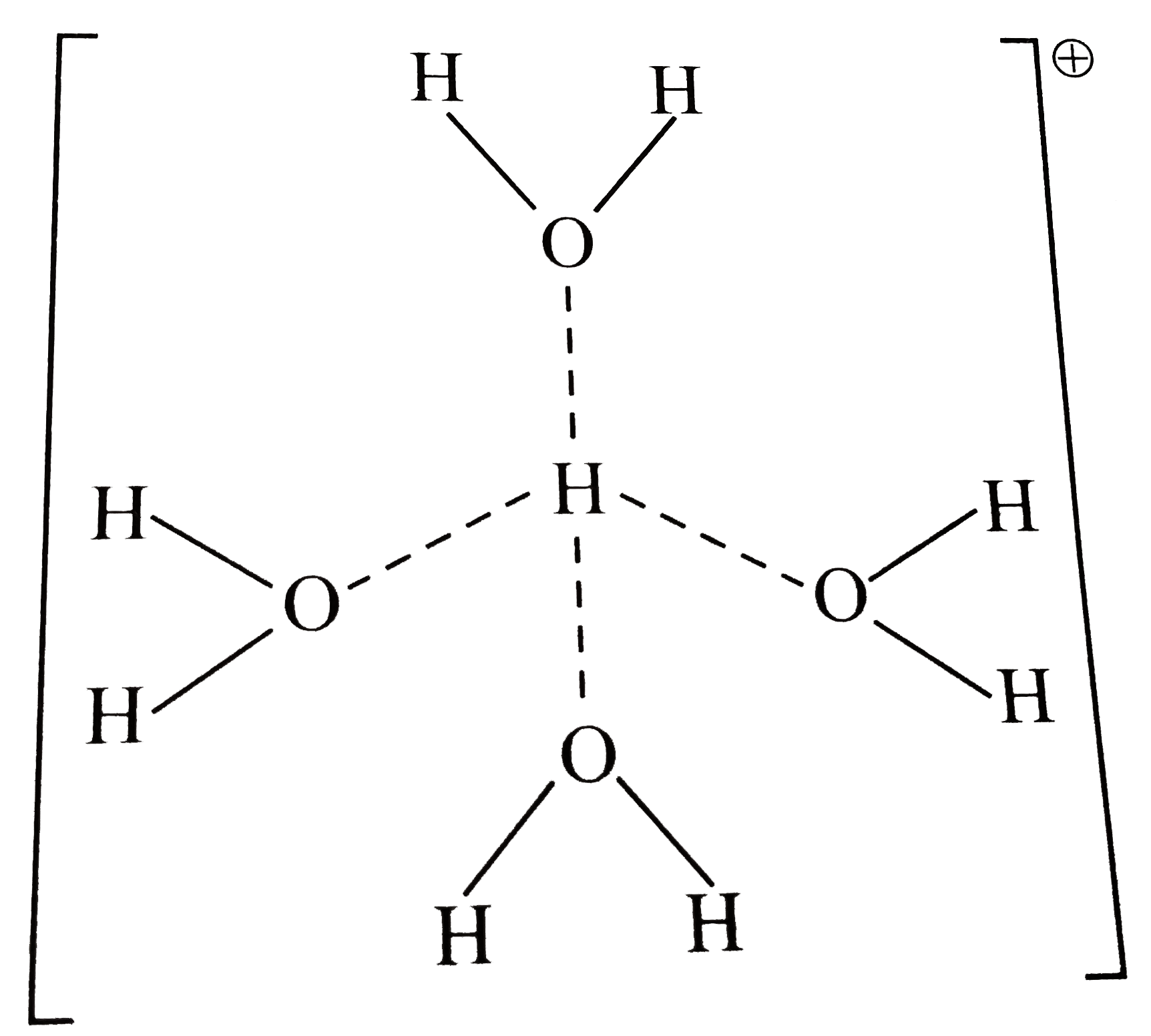
- a. What does [H(9)O(4)]^(o+) stand for ? Draw its structures. b. Can...
Text Solution
|
- H(2)O(2) is a better oxidising agents than water.
Text Solution
|
- The mixture of hydrazine and hydrogen peroxides with a copper (II) cat...
Text Solution
|
- a. When H(2)O(2) is added to blood, rapid evolution of a gas occurs. W...
Text Solution
|